Identification of novel genes responsible for a pollen killer present in local natural populations of Arabidopsis thaliana
- PMID: 39804925
- PMCID: PMC11761171
- DOI: 10.1371/journal.pgen.1011451
Identification of novel genes responsible for a pollen killer present in local natural populations of Arabidopsis thaliana
Abstract
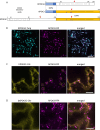
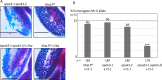
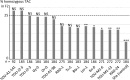
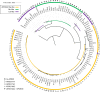
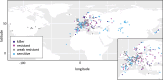
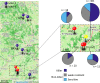
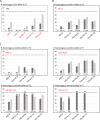
Gamete killers are genetic loci that distort segregation in the progeny of hybrids because the killer allele promotes the elimination of the gametes that carry the sensitive allele. They are widely distributed in eukaryotes and are important for understanding genome evolution and speciation. We had previously identified a pollen killer in hybrids between two distant natural accessions of Arabidopsis thaliana. This pollen killer involves three genetically linked genes, and we previously reported the identification of the gene encoding the antidote that protects pollen grains from the killer activity. In this study, we identified the two other genes of the pollen killer by using CRISPR-Cas9 induced mutants. These two genes are necessary for the killer activity that we demonstrated to be specific to pollen. The cellular localization of the pollen killer encoded proteins suggests that the pollen killer activity involves the mitochondria. Sequence analyses reveal predicted domains from the same families in the killer proteins. In addition, the C-terminal half of one of the killer proteins is identical to the antidote, and one amino acid, crucial for the antidote activity, is also essential for the killer function. Investigating more than 700 worldwide accessions of A. thaliana, we confirmed that the locus is subject to important structural rearrangements and copy number variation. By exploiting available de novo genomic sequences, we propose a scenario for the emergence of this pollen killer in A. thaliana. Furthermore, we report the co-occurrence and behavior of killer and sensitive genotypes in several local populations, a prerequisite for studying gamete killer evolution in the wild. This highlights the potential of the Arabidopsis model not only for functional studies of gamete killers but also for investigating their evolutionary trajectories at complementary geographical scales.
Copyright: © 2025 Ricou et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
APOK3, a pollen killer antidote in Arabidopsis thaliana.Genetics. 2022 Jul 30;221(4):iyac089. doi: 10.1093/genetics/iyac089. Genetics. 2022. PMID: 35666201 Free PMC article.
-
Adaptive reduction of male gamete number in the selfing plant Arabidopsis thaliana.Nat Commun. 2020 Jun 8;11(1):2885. doi: 10.1038/s41467-020-16679-7. Nat Commun. 2020. PMID: 32514036 Free PMC article.
-
Overriding Mendelian inheritance in Arabidopsis with a CRISPR toxin-antidote gene drive that impairs pollen germination.Nat Plants. 2024 Jun;10(6):910-922. doi: 10.1038/s41477-024-01692-1. Epub 2024 Jun 17. Nat Plants. 2024. PMID: 38886523
-
Identification and Evolution of Functional Alleles of the Previously Described Pollen Specific Myrosinase Pseudogene AtTGG6 in Arabidopsis thaliana.Int J Mol Sci. 2016 Feb 22;17(2):262. doi: 10.3390/ijms17020262. Int J Mol Sci. 2016. PMID: 26907263 Free PMC article.
-
Genomic Conflicts that Cause Pollen Mortality and Raise Reproductive Barriers in Arabidopsis thaliana.Genetics. 2016 Jul;203(3):1353-67. doi: 10.1534/genetics.115.183707. Epub 2016 May 6. Genetics. 2016. PMID: 27182945 Free PMC article.
Cited by
-
The evolutionary dynamics of organellar pan-genomes in Arabidopsis thaliana.Genome Biol. 2025 Aug 11;26(1):240. doi: 10.1186/s13059-025-03717-0. Genome Biol. 2025. PMID: 40790212 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

