Senescent Cell-Derived Extracellular Vesicles Inhibit Cancer Recurrence by Coordinating Immune Surveillance
- PMID: 39804967
- PMCID: PMC11878441
- DOI: 10.1158/0008-5472.CAN-24-0875
Senescent Cell-Derived Extracellular Vesicles Inhibit Cancer Recurrence by Coordinating Immune Surveillance
Abstract
Senescence is a nonproliferative survival state that cancer cells can enter to escape therapy. In addition to soluble factors, senescence cells secrete extracellular vesicles (EV), which are important mediators of intercellular communication. To explore the role of senescent cell (SC)-derived EVs (senEV) in inflammatory responses to senescence, we developed an engraftment-based senescence model in wild-type mice and genetically blocked senEV release in vivo, without significantly affecting soluble mediators. SenEVs were both necessary and sufficient to trigger immune-mediated clearance of SCs, thereby suppressing tumor growth. In the absence of senEVs, the recruitment of MHC-II+ antigen-presenting cells (APC) to the senescence microenvironment was markedly impaired. Blocking senEV release redirected the primary target of SC signaling from APCs to neutrophils. Comprehensive transcriptional and proteomic analyses identified six ligands specific to senEVs, highlighting their role in promoting APC-T cell adhesion and synapse formation. APCs activated CCR2+CD4+ TH17 cells, which seemed to inhibit B-cell activation, and CD4+ T cells were essential for preventing tumor recurrence. These findings suggest that senEVs complement the activity of secreted inflammatory mediators by recruiting and activating distinct immune cell subsets, thereby enhancing the efficient clearance of SCs. These conclusions may have implications not only for tumor recurrence but also for understanding senescence during de novo carcinogenesis. Consequently, this work could inform the development of early detection strategies for cancer based on the biology of cellular senescence. Significance: Chemotherapy-treated senescent tumor cells release extracellular vesicles that trigger an immune response and suppress tumor recurrence. See related commentary by Almeida and Melo, p. 833.
©2025 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures
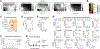
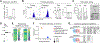

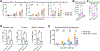

References
-
- Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, and Alimonti A, Cellular Senescence: Aging, Cancer, and Injury. Physiol Rev, 2019. 99(2): p. 1047–1078. - PubMed
-
- Hernandez-Segura A, Nehme J, and Demaria M, Hallmarks of Cellular Senescence, in Trends in Cell Biology. 2018, Elsevier Ltd. p. 436–453. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

