Supervised Exercise for Patients With Metastatic Breast Cancer: A Cost-Utility Analysis Alongside the PREFERABLE-EFFECT Randomized Controlled Trial
- PMID: 39805062
- PMCID: PMC11974635
- DOI: 10.1200/JCO-24-01441
Supervised Exercise for Patients With Metastatic Breast Cancer: A Cost-Utility Analysis Alongside the PREFERABLE-EFFECT Randomized Controlled Trial
Abstract
Purpose: To evaluate the cost utility of a 9-month supervised exercise program for patients with metastatic breast cancer (mBC), compared with control (usual care, supplemented with general activity advice and an activity tracker). Evidence on the cost-effectiveness of exercise for patients with mBC is essential for implementation in clinical practice and is currently lacking.
Methods: A cost-utility analysis was performed alongside the multinational PREFERABLE-EFFECT randomized controlled trial, conducted in 8 centers across Europe and Australia. Patients with mBC (N = 357) were randomly assigned to either a 9-month, twice-weekly, supervised exercise group (EG) or control group (CG). Costs of the exercise program were calculated through a bottom-up approach. Other health care resource use, productivity losses, and quality of life were collected using country-adapted, self-reported questionnaires. Analyses were conducted from a societal perspective with a time horizon of 9 months. Costs were collected and reported in 2021 Euros (€1 = $1.18 US dollars).
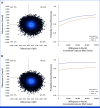
Results: Compared with the CG, EG resulted in a quality-adjusted life-year (QALY) gain of 0.013 (95% CI, -0.02 to 0.05) over a 9-month period. The mean costs of the exercise program were €1,696 per patient with one-on-one supervision (scenario 1) and €609 with one-on-four supervision (scenario 2). These costs were offset by savings in health care and productivity costs, resulting in mean total cost differences of -€163 (scenario 1) and -€1,249 (scenario 2) in favor of EG. The probability of supervised exercise being cost-effective was 65% in scenario 1 and 91% in scenario 2 at a willingness-to-pay threshold of €20,000 per QALY.
Conclusion: Exercise for patients with mBC increases quality of life, decreases costs, and is likely to be cost-effective. Group-based supervision is expected to have even higher cost-savings. Our positive findings can inform reimbursement of supervised exercise interventions for patients with mBC.
Trial registration: ClinicalTrials.gov NCT04120298.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Swain SM, Miles D, Kim S, et al. : Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 21:519-530, 2020 - PubMed
-
- Cortes J, Rugo HS, Cescon DW, et al. : Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med 387:217-226, 2022 - PubMed
-
- Hortobagyi GN, Stemmer SM, Burris HA, et al. : Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med 386:942-950, 2022 - PubMed
-
- Cardoso F, Spence D, Mertz S, et al. : Global analysis of advanced/metastatic breast cancer: Decade report (2005-2015). Breast 39:131-138, 2018 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

