Measurement Properties of the Friedreich Ataxia Rating Scale in Patients with Spinocerebellar Ataxia
- PMID: 39806095
- PMCID: PMC11906947
- DOI: 10.1007/s40120-024-00708-4
Measurement Properties of the Friedreich Ataxia Rating Scale in Patients with Spinocerebellar Ataxia
Abstract
Introduction: The Friedreich Ataxia Rating Scale-Activities of Daily Living (FARS-ADL) is a valid, highly utilized measure for assessing ADL impacts in patients with Friedreich ataxia. We provide evidence of the psychometric validity of the FARS-ADL in two cohorts of patients with spinocerebellar ataxia (SCA).
Methods: Using data from a cohort of real-world subjects with SCA (recruited at Massachusetts General Hospital [MGH]; n = 33) and a phase 3 trial of troriluzole in adults with SCA (NCT03701399 [Study 206]; n = 217), comprising a subset of patients with the SCA3 genotype (n = 89), the psychometric measurement properties and minimal change thresholds of the FARS-ADL were examined.
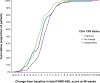
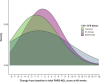
Results: Ceiling effects for the FARS-ADL were absent within the MGH cohort while floor effects were observed for eight of nine items. Excellent internal consistency reliability was observed (αtotal = 0.88; αitems-removed = 0.86-0.87), and item-to-total correlations were acceptable (r = 0.55-0.89 per item). Convergent and divergent validity were supported with strong correlations demonstrated between FARS-ADL and scales measuring similar concepts (Neuro-QOL [Upper], Neuro-QOL [Lower], PROM-ADL, PROM-PHYS, and FARS-FUNC; all P < 0.001) and weaker correlations shown between measures of differing constructs. A two- to three-point threshold for meaningful changes was supported as 0.5 × SD = 2.43, SEM = 2.19. Mean changes from baseline for subjects classified as "improved," "no change," or "deteriorated" were -0.54, 0.22, and 1.47, respectively. Similar trends were observed in the Study 206 all-SCA and SCA3 cohorts.
Conclusion: Psychometric evaluation showed that the FARS-ADL performed well on analyses examining the reliability and validity of the measure and can detect meaningful changes in patients with SCA, including those with SCA3.
Trial registration: ClinicalTrials.gov identifier, NCT03701399 (Study 206).
Keywords: Activities of daily living; Outcomes assessment; Psychometrics; Spinocerebellar ataxias; Validation study.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Michele Potashman, Ainsley Mackenzie, Melissa Wolfe Beiner, Vlad Coric, and Gilbert L’Italien are employees of Biohaven Pharmaceuticals, Inc. Evan Popoff and Lauren Powell are employees of Broadstreet HEOR, which received funds from Biohaven Pharmaceuticals, Inc, for this work. Sub Subramony has received research support from the National Ataxia Foundation, Biohaven Pharmaceuticals, Inc, National Institutes of Health, US Food and Drug Administration, Muscular Dystrophy Association, Wyck Foundation, Friedreich’s Ataxia Research Alliance, Reata, PTC Therapeutics, Retrotope, Avidity Biosciences, Fulcrum Therapeutics, Reneo Pharma, and AavantiBio; and has served on scientific advisory boards for Reata, Avidity, and Dyne Therapeutics. Matthis Synofzik has received consultancy honoraria from Ionis, UCB, Prevail, Orphazyme, Servier, Reata, GenOrph, AviadoBio, Biohaven, Solaxa, Zevra, and Lilly. Jeremy Schmahmann consults for Biohaven Pharmaceuticals and is site PI for Biohaven Pharmaceuticals clinical trials NCT03701399, NCT02960893, and NCT03952806; receives royalties from Oxford University Press, Elsevier, MacKeith Press, and Springer; and is the inventor of the Brief Ataxia Rating Scale, Cerebellar Cognitive Affective/Schmahmann Syndrome Scale, the Patient-Reported Outcome Measure of Ataxia, and the Cerebellar Neuropsychiatry Rating Scale, which are licensed to the General Hospital Corporation. He is supported in part by the National Ataxia Foundation, the MINDLink Foundation, and the Raynor Cerebellum Project. Ethical Approval: Study 206 (BHV4157-206; NCT03701399) was a multisite study approved by a centralized independent Institutional Review Board (Institutional Review Board; Advarra, Columbia, MD, USA), with additional local Institutional Review Board approvals obtained where requested (per institute). The PROM-Ataxia study (MGH psychometric cohort) was approved by Mass General Brigham Human Research Committee Institutional Review Board (Somerville, MA, USA). Both studies were conducted in accordance with the 1964 Declaration of Helsinki.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical

