Venous compression causes chronic cerebral ischaemia in normal pressure hydrocephalus patients
- PMID: 39806498
- PMCID: PMC11731173
- DOI: 10.1186/s12987-024-00608-7
Venous compression causes chronic cerebral ischaemia in normal pressure hydrocephalus patients
Abstract
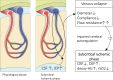
Background: Cerebral autoregulation is a robust regulatory mechanism that stabilizes cerebral blood flow in response to reduced blood pressure, thereby preventing cerebral ischaemia. Scientists have long believed that cerebral autoregulation also stabilizes cerebral blood flow against increases in intracranial pressure, which is another component that determines cerebral perfusion pressure. However, this idea was inconsistent with the complex pathogenesis of normal pressure hydrocephalus, which includes components of chronic cerebral ischaemia due to mild increases in intracranial pressure.
Methods: Twenty-one patients who underwent ventriculoperitoneal shunt surgery for normal pressure hydrocephalus were included in this study. To determine the pressure setting of the Codman-Hakim programmable valve, intracranial pressure was measured after shunt surgery by puncturing the Ommaya reservoir, which formed a closed circuit with the needle and the syringe. Then, intracranial pressure was continuously measured with intermittent infusion of cerebrospinal fluid from the same closed circuit. We also continuously measured oximetry data, such as regional cerebral oxygen saturation derived from near-infrared spectroscopy monitoring. These data were digitized, recorded, and used for calculating intracranial compliance and the relationship between cerebrospinal fluid volume loading and intracranial pressure.
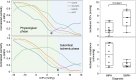
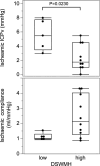
Results: This study demonstrates that in patients with normal pressure hydrocephalus, cerebral venous vascular bed compression induces mild cerebral ischaemia when intracranial pressure is slightly higher than physiological venous pressure. Cerebral venous compression impairs cerebral blood flow by quadratically increasing circulatory resistance according to Poiseuille's law. Furthermore, chronic cerebral ischaemia occurred even at low or normal intracranial pressures when deep and subcortical white matter hyperintensities (DSWMHs) were severe.
Conclusion: The fact that cerebral blood flow impairment begins at very low intracranial pressures indicates that cerebral autoregulation to compensate for reduced venous blood flow is not functioning adequately in NPH. These processes provide a link between impaired cerebrospinal fluid circulation, cerebral autoregulation, and neurological dysfunction, which has been missing in patients with NPH involving small vessel arteriosclerosis. These findings may provide insight into similar conditions, such as normal-tension glaucoma and chronic kidney disease, in which a mild increase in local compartment pressure leads to chronic ischemia in organs protected by autoregulatory mechanisms.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Consent for publication: I confirm the corresponding author has read the journal policies and submit this manuscript in accordance with those policies. Competing interests: The authors declare no competing interests.
Figures



Comment in
-
Is the ischemia found in normal pressure hydrocephalus secondary to venous compression or arterial constriction? A comment on Ohmura et al.Fluids Barriers CNS. 2025 Mar 19;22(1):29. doi: 10.1186/s12987-025-00640-1. Fluids Barriers CNS. 2025. PMID: 40108646 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

