Protocol for a multisite randomised controlled trial assessing the effect of the Telehealth for Early Developmental Intervention in babies born very preterm (TEDI-Prem) programme on neurodevelopmental outcomes and parent well-being
- PMID: 39806618
- PMCID: PMC11667297
- DOI: 10.1136/bmjopen-2024-086904
Protocol for a multisite randomised controlled trial assessing the effect of the Telehealth for Early Developmental Intervention in babies born very preterm (TEDI-Prem) programme on neurodevelopmental outcomes and parent well-being
Erratum in
-
Correction for 'Protocol for a multisite randomised controlled trial assessing the effect of the Telehealth for early developmental Intervention in babies born very preterm (TEDI-Prem) programme on neurodevelopmental outcomes and parent well-being'.BMJ Open. 2025 Jan 15;15(1):e086904corr1. doi: 10.1136/bmjopen-2024-086904corr1. BMJ Open. 2025. PMID: 39819960 Free PMC article. No abstract available.
Abstract
Introduction: Infants born very preterm (VPT, <32 weeks' gestation) are at increased risk for neurodevelopmental impairments including motor, cognitive and behavioural delay. Parents of infants born VPT also have poorer mental health outcomes compared with parents of infants born at term.We have developed an intervention programme called TEDI-Prem (Telehealth for Early Developmental Intervention in babies born very preterm) based on previous research. TEDI-Prem aims to improve neurodevelopmental outcomes and parental well-being in children born VPT. Here we present the protocol outlining a multicentre, pragmatic, parallel-group, randomised controlled trial to determine the efficacy of TEDI-Prem plus usual care, compared with usual care alone.
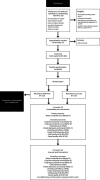
Methods and analysis: We will recruit 466 VPT infants from the neonatal units of five hospitals in Victoria, Australia. Participants will be randomised, stratified by site of recruitment and multiple births, to TEDI-Prem plus usual care or usual care alone. The TEDI-Prem intervention programme involves 13 sessions across three phases. Phase 1 commences in the neonatal unit with four face-to-face sessions with parent/s and a physiotherapist/occupational therapist. Once discharged from the hospital, sessions across phases 2 and 3 (six and three sessions, respectively) continue via telehealth until infants are 12 months' corrected age (CA).The primary outcome is the Bayley Scales of Infant and Toddler Development-fourth edition (Bayley-4) Motor Composite Score at 12 months' CA. Secondary outcomes address other neurodevelopmental domains (Bayley-4 cognitive and language composite score; Infant Toddler Social Emotional Assessment), parental mental health (Depression Anxiety and Stress Scale 21), parent-child interaction (Emotional Availability Scale) and programme cost-effectiveness which encompasses parent quality of life (Short-Form Six-Dimension Quality of Life) and child quality of life (EuroQol Toddler and Infant Populations measure) at 12 and 24 months' CA.Mean differences between groups will be examined using linear regression for continuous outcomes and logistic regression for binary outcomes. All models will be fitted via generalised estimating equations to account for multiple births and adjusted for the hospital sites.
Ethics and dissemination: This trial has Royal Children's Hospital Human Research and Ethics Committee approval (HREC/67604/RCHM-2020) with specific site approval for all participating sites. Findings will be disseminated through peer-reviewed publications, conference presentations, digital and print media and to participants.
Trial egistration number: This trial is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12621000364875).
Keywords: Clinical Trial; Developmental neurology & neurodisability; Neonatal intensive & critical care; Telemedicine.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Australian Institute of Health Welfare . Australia’s Mothers and Babies 2017—in Brief. Canberra: AIHW; 2019.
