Enhancing the Implant Osteointegration via Supramolecular Co-Assembly Coating with Early Immunomodulation and Cell Colonization
- PMID: 39806935
- PMCID: PMC11884616
- DOI: 10.1002/advs.202410595
Enhancing the Implant Osteointegration via Supramolecular Co-Assembly Coating with Early Immunomodulation and Cell Colonization
Abstract
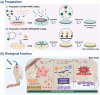
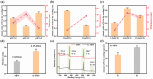
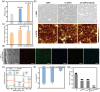
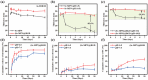
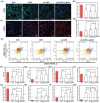
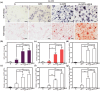
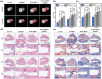
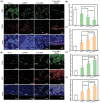
Osteointegration, the effective coupling between an implant and bone tissue, is a highly intricate biological process. The initial stages of bone-related immunomodulation and cellular colonization play crucial roles, but have received limited attention. Herein, a novel supramolecular co-assembled coating of strontium (Sr)-doped metal polyphenol networks (MPN) modified with c(RGDfc) is developed and well-characterized, for eliciting an early immunomodulation and cellular colonization. The results showed that the (Sr-MPN)@RGD coating significantly regulated the polarization of macrophages to the M2 phenotype by controllable release of Sr, and promote the initial adhesion of bone marrow mesenchymal stem cells (BMSCs) by RGD presented on MPN. Notably, the (Sr-MPN)@RGD attenuated osteoclast differentiation and oxidative stress as well as enhanced osteoblast differentiation and angiogenesis due to macrophage polarization toward M2 phenotype, which in turn has a profound effect on neighboring cells through paracrine signaling. In vivo results showed that the (Sr-MPN)@RGD coating manifested superior osseointegration and bone maturation to the bare Ti-rod or Ti-rod coated with MPN and Sr-MPN. This work contributed to the design of multifunctional implant coatings that address the complex biological process of osteointegration from the perspective of orchestrating stem cell recruitment with immunomodulatory strategies.
Keywords: RGD; colonization; metal‐phenolic network; osteoimmunology; strontium.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
