Novel gel-immersion endoscopic injection sclerotherapy method for prophylactic hemostasis of esophageal varices: A pilot feasibility and safety study (with video)
- PMID: 39807430
- PMCID: PMC11726624
- DOI: 10.1002/deo2.70056
Novel gel-immersion endoscopic injection sclerotherapy method for prophylactic hemostasis of esophageal varices: A pilot feasibility and safety study (with video)
Abstract
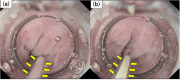
Endoscopic injection sclerotherapy (EIS) is a useful prophylactic hemostatic procedure for esophageal varices. However, injecting sclerosing agents into blood vessels is technically challenging and often ineffective. Gel-immersion EIS (GI-EIS) may facilitate easier intravascular sclerosing agent injection by dilating the varices and enhancing scope stability by maintaining low intra-gastrointestinal pressure. Therefore, we aimed to evaluate the effectiveness and safety of this procedure. This retrospective study included 18 patients (14 men and four women; median age, 70 years; age range, 18-83 years) who underwent GI-EIS at Osaka Medical Pharmaceutical University Hospital between December 1, 2022, and January 30, 2024. Patients who were at least 18 years of age at the time of treatment were included. No patients were excluded from the study. Thirty-four punctures were performed. The donor vessel angiography success rate was 88.2% (30 of 34 punctures). The clinical success rate was 94.4% (17 of 18 patients). Esophageal varices in most patients disappeared or were reduced by 1 month after treatment. Adverse events related to the procedure included fever (three patients) and chest pain (one patient); however, both were resolved with conservative treatment. No respiratory deterioration due to aspiration occurred during the procedure. The results of this study demonstrate that GI-EIS is a safe, clinically feasible, and effective treatment option for prophylactic hemostasis of esophageal varices.
Keywords: digestive system endoscopy; endoscopic injection sclerotherapy; esophageal varices; gel immersion endoscopy; prophylactic hemostasis.
© 2025 The Author(s). DEN Open published by John Wiley & Sons Australia, Ltd on behalf of Japan Gastroenterological Endoscopy Society.
Conflict of interest statement
None.
Figures


References
-
- Jensen DM. Endoscopic screening for varices in cirrhosis: Findings, implications, and outcomes. Gastroenterology 2002; 122: 1620–1630. - PubMed
-
- Masaki M, Obara K, Suzuki S et al. The destructive effects of sclerosant ethanolamine oleate on mammalian vessel endothelium. Gastroenterol Jpn 1990; 25: 230–235. - PubMed
-
- Yuki M, Kazumori H, Yamamoto S, Shizuku T, Kinoshita Y. Prognosis following endoscopic injection sclerotherapy for esophageal varices in adults: 20‐year follow‐up study. Scand J Gastroenterol 2008; 43: 1269–1274. - PubMed
LinkOut - more resources
Full Text Sources
