Inhibition of IFITM3 in cerebrovascular endothelium alleviates Alzheimer's-related phenotypes
- PMID: 39807629
- PMCID: PMC11851164
- DOI: 10.1002/alz.14543
Inhibition of IFITM3 in cerebrovascular endothelium alleviates Alzheimer's-related phenotypes
Abstract
Introduction: Interferon-induced transmembrane protein 3 (IFITM3) modulates γ-secretase in Alzheimer's Disease (AD). Although IFITM3 knockout reduces amyloid β protein (Aβ) production, its cell-specific effect on AD remains unclear.
Methods: Single nucleus RNA sequencing (snRNA-seq) was used to assess IFITM3 expression. Adeno-associated virus-BI30 (AAV-BI30) was injected to reduce IFITM3 expression in the cerebrovascular endothelial cells (CVECs). The effects on AD phenotypes in cells and AD mice were examined through behavioral tests, two-photon imaging, flow cytometry, Western blot, immunohistochemistry, and quantitative polymerase chain reaction assay (qPCR).
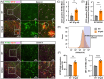
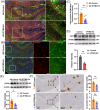
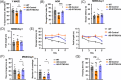
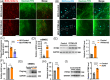
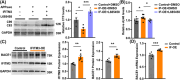
Results: IFITM3 expression was increased in the CVECs of patients with AD. Overexpression of IFITM3 in primary endothelial cells enhanced Aβ generation through regulating beta-site APP cleaving enzyme 1 (BACE1) and γ-secretase. Aβ further increased IFITM3 expression, creating a vicious cycle. Knockdown of IFITM3 in CVECs decreased Aβ accumulation within cerebrovascular walls, reduced Alzheimer's-related pathology, and improved cognitive performance in AD transgenic mice.
Discussion: Knockdown of IFITM3 in CVECs alleviates AD pathology and cognitive impairment. Targeting cerebrovascular endothelial IFITM3 holds promise for AD treatment.
Highlights: Interferon-induced transmembrane protein 3 (IFITM3) expression was increased in the cerebrovascular endothelial cells (CVECs) of patients with Alzheimer's Disease (AD). Cerebrovascular endothelial IFITM3 regulates amyloid β protein (Aβ) generation through regulating beta-site APP cleaving enzyme 1 (BACE1) and γ-secretase. Knockdown of IFITM3 in CVECs reduces Aβ deposits and improves cognitive impairments in AD transgenic mice. Cerebrovascular endothelial IFITM3 could be a potential target for the treatment of AD.
Keywords: Alzheimer's Disease; Aβ; IFITM3; cerebrovascular endothelium; cognitive impairment.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no competing interests. Author disclosures are available in the supporting information.
Figures


References
-
- Peng L, Bestard‐Lorigados I, Song W. The synapse as a treatment avenue for Alzheimer's Disease. Mol Psychiatry. 2022;27:2940‐2949. - PubMed
-
- van de Haar HJ, Jansen JFA, van Osch MJP, et al. Neurovascular unit impairment in early Alzheimer's disease measured with magnetic resonance imaging. Neurobiol Aging. 2016;45:190‐196. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

