Efficacy of Neoadjuvant Radiotherapy After Chemotherapy and the Optimal Interval from Radiotherapy to Surgery for Borderline Resectable and Resectable Pancreatic Cancer
- PMID: 39808212
- PMCID: PMC11882644
- DOI: 10.1245/s10434-024-16743-2
Efficacy of Neoadjuvant Radiotherapy After Chemotherapy and the Optimal Interval from Radiotherapy to Surgery for Borderline Resectable and Resectable Pancreatic Cancer
Abstract
Background: Benefits of neoadjuvant treatment for pancreatic cancer with major vessel invasion has been demonstrated through randomized controlled trials; however, the optimal neoadjuvant treatment strategy remains controversial, especially for radiotherapy. Therefore, we aimed to evaluate the efficacy and safety of neoadjuvant radiotherapy followed by chemotherapy and the optimal time interval to undergo surgery after radiotherapy in (borderline) resectable pancreatic cancer.
Methods: Between 2013 and 2022, patients with (borderline) resectable pancreatic cancer with vessel contact who received 5-fluorouracil with leucovorin, oxaliplatin, and irinotecan or gemcitabine and nanoparticle albumin-bound paclitaxel as initial treatment following surgery were included. Patients who received radiotherapy after chemotherapy and those who did not were matched using 1:1 nearest-neighbor propensity scores. Propensity scores were measured using the tumor size at initial image, duration of neoadjuvant chemotherapy, and responsiveness to neoadjuvant chemotherapy.
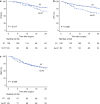
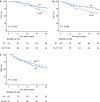
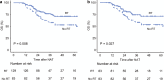
Results: Of 212 patients, 166 patients were retrieved for the matched cohort. Patients who received radiotherapy had significantly better postoperative survival, local control, and R0 resection rates than those who did not. Furthermore, patients who underwent surgery within 4 weeks after completing radiotherapy had lower intraoperative blood loss and a clinically relevant postoperative pancreatic fistula rate than those who underwent surgery after more than 4 weeks.
Conclusions: In patients with (borderline) resectable pancreatic cancer with vessel contact who were scheduled for curative-intent surgery after neoadjuvant chemotherapy, additional radiotherapy was associated with better postoperative survival and local control. Furthermore, our findings suggested that scheduling surgery within 4 weeks following radiation therapy might enhance the perioperative outcomes.
Keywords: Neoadjuvant chemotherapy; Neoadjuvant radiotherapy; Pancreatectomy; Pancreatic cancer; Stereotactic body radiation therapy.
© 2025. The Author(s).
Conflict of interest statement
Disclosure: Won-Gun Yun, Yoon Soo Chae, Youngmin Han, Hye-Sol Jung, Young Jae Cho, Hyun-Cheol Kang, Wooil Kwon, Joon Seong Park, Eui Kyu Chie, and Jin-Young Jang declare no competing interests that may be relevant to the contents of this study.
Figures
Similar articles
-
Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial.BMC Cancer. 2021 Mar 23;21(1):300. doi: 10.1186/s12885-021-08031-z. BMC Cancer. 2021. PMID: 33757440 Free PMC article.
-
Neoadjuvant therapy versus upfront surgery for borderline-resectable pancreatic cancer.Minerva Chir. 2020 Feb;75(1):15-24. doi: 10.23736/S0026-4733.19.07958-6. Epub 2019 May 20. Minerva Chir. 2020. PMID: 31115240
-
Resectable Pancreatic Cancer with CA19-9 > 500 U/mL: A Biological Indicator for Survival Benefit with Intensive Neoadjuvant Chemotherapy.Ann Surg Oncol. 2025 Aug;32(8):5411-5420. doi: 10.1245/s10434-025-17407-5. Epub 2025 May 13. Ann Surg Oncol. 2025. PMID: 40358779
-
Neoadjuvant Treatment for Borderline Resectable Pancreatic Ductal Adenocarcinoma.Dig Surg. 2019;36(6):455-461. doi: 10.1159/000493466. Epub 2018 Nov 8. Dig Surg. 2019. PMID: 30408790 Review.
-
Neoadjuvant therapy for pancreas cancer: Global perspective and optimal care pathways in low to middle-income countries.J Surg Oncol. 2021 May;123(6):1441-1448. doi: 10.1002/jso.26365. J Surg Oncol. 2021. PMID: 33831251 Review.
Cited by
-
[Stereotactic radiotherapy in functionally inoperable but technically resectable pancreatic cancer].Strahlenther Onkol. 2025 Aug;201(8):857-858. doi: 10.1007/s00066-025-02429-5. Epub 2025 Jun 17. Strahlenther Onkol. 2025. PMID: 40526131 German. No abstract available.
-
Hybrid Systems of Gels and Nanoparticles for Cancer Therapy: Advances in Multifunctional Therapeutic Platforms.Gels. 2025 Feb 26;11(3):170. doi: 10.3390/gels11030170. Gels. 2025. PMID: 40136875 Free PMC article. Review.
-
ASO Author Reflections: Is There an Extra Benefit of Adding Neoadjuvant Radiotherapy to Chemotherapy in Patients with (Borderline) Resectable Pancreatic Cancer?Ann Surg Oncol. 2025 Apr;32(4):2859-2860. doi: 10.1245/s10434-024-16829-x. Epub 2025 Jan 15. Ann Surg Oncol. 2025. PMID: 39812917 Free PMC article. No abstract available.
-
Evaluation of feasibility and clinical outcomes of robot-assisted pancreaticoduodenectomy after neoadjuvant treatment for patients with advanced pancreatic ductal adenocarcinoma: a retrospective propensity score-matched cohort study.Ann Surg Treat Res. 2025 Aug;109(2):61-70. doi: 10.4174/astr.2025.109.2.61. Epub 2025 Jul 30. Ann Surg Treat Res. 2025. PMID: 40785812 Free PMC article.
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. - PubMed
-
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–406. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

