Cisplatin Exposure Dysregulates Insulin Secretion in Male and Female Mice
- PMID: 39808439
- PMCID: PMC11926276
- DOI: 10.2337/db24-0419
Cisplatin Exposure Dysregulates Insulin Secretion in Male and Female Mice
Abstract
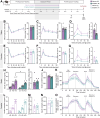
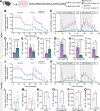
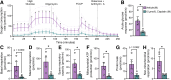
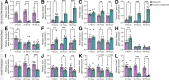
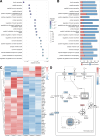
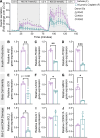
Cancer survivors who receive cisplatin chemotherapy have an increased risk of type 2 diabetes, but the underlying mechanisms remain unclear. The aim of this study was to investigate whether cisplatin impacts β-cell health and function, thereby contributing to increased type 2 diabetes risk in cancer survivors. In vivo and in vitro cisplatin exposure dysregulated insulin secretion in male and female mice. In vitro cisplatin exposure reduced oxygen consumption, impaired β-cell exocytotic capacity, and altered expression of genes within the insulin secretion pathway in mouse islets. Understanding how chemotherapeutic drugs cause β-cell injury is critical for designing targeted interventions to reduce the risk of cancer survivors developing type 2 diabetes after treatment.
© 2025 by the American Diabetes Association.
Conflict of interest statement
Figures


References
-
- Lega IC, Pole JD, Austin PC, Lau C, Nathan PC, Baxter NN.. Diabetes risk in childhood cancer survivors: a population-based study. Can J Diabetes 2018;42:533–539 - PubMed
-
- Singh S, Earle CC, Bae SJ, et al. Incidence of diabetes in colorectal cancer survivors. J Natl Cancer Inst 2016;108:djv402. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

