An activin receptor-like kinase 1-governed monocytic lineage shapes an immunosuppressive landscape in breast cancer metastases
- PMID: 39808498
- PMCID: PMC11870737
- DOI: 10.1172/JCI183086
An activin receptor-like kinase 1-governed monocytic lineage shapes an immunosuppressive landscape in breast cancer metastases
Abstract
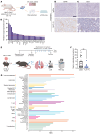
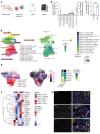
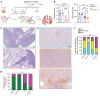
The biology centered around the TGF-β type I receptor activin receptor-like kinase (ALK) 1 (encoded by ACVRL1) has been almost exclusively based on its reported endothelial expression pattern since its first functional characterization more than 2 decades ago. Here, in efforts to better define the therapeutic context in which to use ALK1 inhibitors, we uncover a population of tumor-associated macrophages (TAMs) that, by virtue of their unanticipated Acvrl1 expression, are effector targets for adjuvant antiangiogenic immunotherapy in mouse models of metastatic breast cancer. The combinatorial benefit depended on ALK1-mediated modulation of the differentiation potential of bone marrow-derived granulocyte-macrophage progenitors, the release of CD14+ monocytes into circulation, and their eventual extravasation. Notably, ACVRL1+ TAMs coincided with an immunosuppressive phenotype and were overrepresented in human cancers progressing on therapy. Accordingly, breast cancer patients with a prominent ACVRL1hi TAM signature exhibited a significantly shorter survival. In conclusion, we shed light on an unexpected multimodal regulation of tumorigenic phenotypes by ALK1 and demonstrate its utility as a target for antiangiogenic immunotherapy.
Keywords: Breast cancer; Cancer immunotherapy; Endothelial cells; Immunology; Oncology.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

