PDGFRα inhibition reduces myofibroblast expansion in the fibrotic rim and enhances recovery after ischemic stroke
- PMID: 39808499
- PMCID: PMC11870733
- DOI: 10.1172/JCI171077
PDGFRα inhibition reduces myofibroblast expansion in the fibrotic rim and enhances recovery after ischemic stroke
Abstract
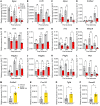
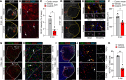
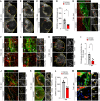
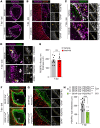
Ischemic stroke is a major cause of disability in adults. Early treatment with thrombolytics and/or thrombectomy can significantly improve outcomes; however, following these acute interventions, treatment is limited to rehabilitation therapies. Thus, identification of therapeutic strategies that can help restore brain function in the post-acute phase remains a major challenge. Here we report that genetic or pharmacologic inhibition of the PDGF-CC/PDGFRα pathway, which has previously been implicated in stroke pathology, significantly reduced myofibroblast expansion in the border of the fibrotic scar and improved outcome in a sensory-motor integration test after experimental ischemic stroke. This was supported by gene expression analyses of cerebrovascular fragments showing upregulation of profibrotic/proinflammatory genes, including genes of the TGF pathway, after ischemic stroke or intracerebroventricular injection of active PDGF-CC. Further, longitudinal intravital 2-photon imaging revealed that inhibition of PDGFRα dampened the biphasic pattern of stroke-induced vascular leakage and enhanced vascular perfusion in the ischemic lesion. Importantly, we found PDGFRα inhibition to be effective in enhancing functional recovery when initiated 24 hours after ischemic stroke. Our data implicate the PDGF-CC/PDGFRα pathway as a crucial mediator modulating post-stroke pathology and suggest a post-acute treatment opportunity for patients with ischemic stroke targeting myofibroblast expansion to foster long-term CNS repair.
Keywords: Fibrosis; Growth factors; Neuroscience; Stroke; Vascular biology.
Conflict of interest statement
Figures


Comment in
- Imatinib on target in stroke recovery doi: 10.1172/JCI190024
References
-
- Saini V, et al. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(20 suppl 2):S6–S16. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

