TRAIL agonists rescue mice from radiation-induced lung, skin, or esophageal injury
- PMID: 39808500
- PMCID: PMC11870730
- DOI: 10.1172/JCI173649
TRAIL agonists rescue mice from radiation-induced lung, skin, or esophageal injury
Abstract
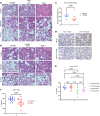
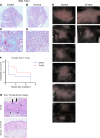
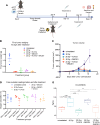
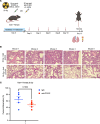
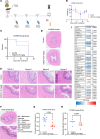
Radiotherapy can be limited by pneumonitis, which is impacted by innate immunity, including pathways regulated by TRAIL death receptor DR5. We investigated whether DR5 agonists could rescue mice from toxic effects of radiation and found that 2 different agonists, parenteral PEGylated trimeric TRAIL (TLY012) and oral TRAIL-inducing compound (TIC10/ONC201), could reduce pneumonitis, alveolar wall thickness, and oxygen desaturation. Lung protection extended to late effects of radiation including less fibrosis at 22 weeks in TLY012-rescued survivors versus unrescued surviving irradiated mice. Wild-type orthotopic breast tumor-bearing mice receiving 20 Gy thoracic radiation were protected from pneumonitis with disappearance of tumors. At the molecular level, radioprotection appeared to be due to inhibition of CCL22, a macrophage-derived chemokine previously associated with radiation pneumonitis and pulmonary fibrosis. Treatment with anti-CCL22 reduced lung injury in vivo but less so than TLY012. Pneumonitis severity was worse in female versus male mice, and this was associated with increased expression of X-linked TLR7. Irradiated mice had reduced esophagitis characterized by reduced epithelial disruption and muscularis externa thickness following treatment with the ONC201 analog ONC212. The discovery that short-term treatment with TRAIL pathway agonists effectively rescues animals from pneumonitis, dermatitis, and esophagitis following high doses of thoracic radiation exposure has important translational implications.
Keywords: Cancer; Innate immunity; Oncology; Radiation therapy.
Conflict of interest statement
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

