mTORC1 regulates the pyrimidine salvage pathway by controlling UCK2 turnover via the CTLH-WDR26 E3 ligase
- PMID: 39808525
- PMCID: PMC11840829
- DOI: 10.1016/j.celrep.2024.115179
mTORC1 regulates the pyrimidine salvage pathway by controlling UCK2 turnover via the CTLH-WDR26 E3 ligase
Abstract
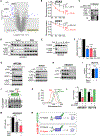
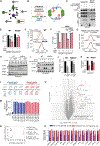
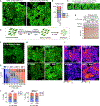
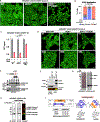
One critical aspect of cell proliferation is increased nucleotide synthesis, including pyrimidines. Pyrimidines are synthesized through de novo and salvage pathways. Prior studies established that the mammalian target of rapamycin complex 1 (mTORC1) promotes pyrimidine synthesis by activating the de novo pathway for cell proliferation. However, the involvement of mTORC1 in regulating the salvage pathway remains unclear. Here, we report that mTORC1 controls the half-life of uridine cytidine kinase 2 (UCK2), the rate-limiting enzyme in the salvage pathway. Specifically, UCK2 is degraded via the CTLH-WDR26 E3 complex during mTORC1 inhibition, which is prevented when mTORC1 is active. We also find that UCK1, an isoform of UCK2, affects the turnover of UCK2 by influencing its cellular localization. Importantly, altered UCK2 levels through the mTORC1-CTLH E3 pathway affect pyrimidine salvage and the efficacy of pyrimidine analog prodrugs. Therefore, mTORC1-CTLH E3-mediated degradation of UCK2 adds another layer of complexity to mTORC1's role in regulating pyrimidine metabolism.
Keywords: CP: Metabolism; CP: Molecular biology; CTLH; UCK2; WDR26; YPEL5; degradomics; mTOR; mTORC1; pyrimidine; pyrimidine salvage; ubiquitin.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

