Salvage autologous transplant in relapsed multiple myeloma: long-term follow-up of the phase 3 GMMG ReLApsE trial
- PMID: 39808798
- PMCID: PMC12060157
- DOI: 10.1182/blood.2024027342
Salvage autologous transplant in relapsed multiple myeloma: long-term follow-up of the phase 3 GMMG ReLApsE trial
Abstract
The multicenter, phase 3 German-Speaking Myeloma Multicenter Group (GMMG) ReLApsE trial randomized patients with relapsed and/or refractory multiple myeloma (RRMM) equally to lenalidomide/dexamethasone (LEN/DEX; 25 mg days 1-21, DEX 40 mg weekly, in 4-week cycles) reinduction, salvage high-dose chemotherapy (sHDCT; melphalan 200 mg/m2), autologous stem cell transplantation (ASCT), and LEN maintenance (10 mg/d; transplant arm, n = 139) vs continuous LEN/DEX (control arm, n = 138). Ninety-four percent of patients had received frontline HDCT/ASCT. We report an updated analysis of survival end points with a median follow-up of 99 months. Median progression-free survival (PFS) was 20.5 and 19.3 months in the transplant and control arm, respectively (hazard ratio [HR], 0.98; P = .9). Median overall survival (OS) was 67.1 and 62.7 months, respectively, (HR 0.89; P = .44). Landmark analyses from sHDCT and the contemporaneous LEN/DEX cycle 5 were performed because of 29% dropout of patients before sHDCT/ASCT in the transplant arm but did not reveal significant differences in PFS/OS. Time to progression after frontline HDCT/ASCT was a prognostic factor but did not predict benefit from sHDCT/ASCT. The GMMG ReLApsE trial does not support use of sHDCT/ASCT in RRMM after frontline HDCT/ASCT. This trial was registered at www.clinicaltrialsregister.eu as #EudraCT2009-013856-61.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.-A.B. reports consultancy with Takeda and Novartis; received honoraria from Takeda, Novartis, Sanofi, and Stemline; received travel support from Celgene, Amgen, Janssen, and Sanofi; and received research funding from Novartis and Sanofi. M.S.R. reports consultancy with, honoraria from, and membership on an entity's board of directors or advisory committees of, Janssen; AbbVie, GlaxoSmithKline (GSK), Bristol Myers Squibb (BMS), Sanofi and Amgen; and received research funding from Janssen, BMS, Sanofi, and Heidelberg Pharma. M.M. reports honoraria from Amgen, Takeda, BMS, Janssen, Stemline, and Roche. E.K.M. reports a consulting or advisory role with Amgen, BMS/Celgene, GSK, Janssen-Cilag, Oncopeptides, Pfizer, Sanofi, Stemline, and Takeda; reports honoraria from Amgen, BMS/Celgene, GSK, Janssen-Cilag, Oncopeptides, Pfizer, Sanofi, Stemline, and Takeda; reports research funding from BMS/Celgene, GSK, Janssen-Cilag, Sanofi, and Takeda; and reports travel accommodations and expenses from BMS/Celgene, GSK, Janssen-Cilag, Sanofi, Stemline, and Takeda. S.K. reports honoraria from Amgen. B.G. reports consultancy/advisory board membership with Roche, Kite, and Novartis; reports grants from, or contracts with, Roche, Kite, and Novartis; received honoraria from Roche, Kite, and Novartis; and received travel support from Roche, Kite, and Novartis. U.G. reports stock and other ownership interests in BioNTech; reports honoraria from Boehringer Ingelheim, Amgen, AstraZeneca, BMS, Merck Sharp & Dohme (MSD) Oncology, Sanofi, Fujifilm, Novartis, Cellrion, and Ipsen; reports consultancy/advisory board membership with Amgen and MSD Oncology; received research funding from Ipsen, MacroGenics; and reports travel support from Boehringer Ingelheim, and GSK. R.F. reports honoraria from Janssen, Amgen, GSK, Takeda, and BMS. M.H. reports consultancy with Novartis, BMS/Celgene, Gilead, Pfizer, Incyte, Sanofi/Aventis, Roche, Amgen, Sobi, and Janssen; and reports honoraria from Novartis, Sobi, Gilead, and Falk Foundation. I.v.M. reports consultancy with Takeda, Pfizer, Sanofi, and BMS. C.S. reports consultancy/advisory board membership with BMS, GSK, Janssen, Pfizer, Roche, and Takeda; reports honoraria from Amgen, BMS, GSK, Janssen, MSD, Novartis, Roche, Sanofi, and Takeda; reports research funding from Janssen and Takeda; and received travel support from BMS, Janssen, Sanofi Aventis, and Takeda. H.J.S. reports consultancy/advisory board membership with Amgen, AstraZeneca, BMS/Celgene, Genzyme, GSK, Janssen-Cilag, Oncopeptides, Pfizer, Sanofi, and Stemline; reports honoraria from AbbVie, Amgen, AstraZeneca, BMS/Celgene, Genzyme, GSK, Janssen-Cilag, Oncopeptides, Pfizer, Roche, Sanofi, Stemline, and Takeda; and received travel support from Amgen, BMS/Celgene, Janssen-Cilag, and Sanofi. B.B. reports honoraria from Janssen-Cilag, Amgen, Sanofi, GSK, and Pfizer; and travel support from Janssen-Cilag, Amgen, and Sanofi. K.C.W. reports consultancy/advisory board membership with AbbVie, Amgen, Adaptive Biotech, BMS/Celgene, BeiGene, Janssen, GSK, Karyopharm, Oncopeptides, Pfizer, Regeneron, Roche Pharma, Sanofi, Takeda, and Menarini; reports honoraria from AbbVie, Amgen, Adaptive Biotech, AstraZeneca, BMS/Celgene, BeiGene, Janssen, GSK, Karyopharm, Novartis, Oncopeptides, Pfizer, Roche Pharma, Sanofi, Stemline, Takeda, and Menarini; and received research funding from AbbVie, Amgen, BMS/Celgene, Janssen, GSK, Pfizer, Sanofi, and Takeda. H.G. reports consultancy/advisory board membership with Amgen, BMS, Janssen, Sanofi, and Adaptive Biotechnology; received honoraria from Amgen, BMS, Chugai, GSK, Janssen, Novartis, Sanofi, and Pfizer; received research funding from Amgen, BMS, Celgene, GlycoMimetics Inc, GSK, Heidelberg Pharma, Hoffmann-La Roche, Karyopharm, Janssen, Incyte Corporation, Millenium Pharmaceuticals Inc, Molecular Partners, Merck Sharp and Dohme, MorphoSys AG, Pfizer, Sanofi, Takeda, and Novartis; received travel support from Amgen, BMS, GSK, Janssen, Novartis, Sanofi, and Pfizer; reports grants from Amgen, Array Biopharma/Pfizer, BMS/Celgene, Chugai, Dietmar Hopp Foundation, Janssen, Johns Hopkins University, Mundipharma GmbH, and Sanofi. The remaining authors declare no competing financial interests.
A list of the German-Speaking Myeloma Multicenter Group trial sites and investigators involved in the ReLApsE trial appears in “Appendix.”
Figures

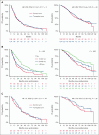
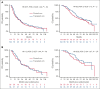
Comment in
-
The decline of transplant for relapsed myeloma.Blood. 2025 Apr 17;145(16):1711-1712. doi: 10.1182/blood.2024028275. Blood. 2025. PMID: 40244643 No abstract available.
References
-
- Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(3):309–322. - PubMed
-
- Kumar SK, Callander NS, Adekola K, et al. Multiple myeloma, version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2023;21(12):1281–1301. - PubMed
-
- Giralt S, Garderet L, Durie B, et al. American Society of Blood and Marrow Transplant, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network and International Myeloma Working Group consensus conference on salvage hematopoietic cell transplant. Biol Blood Marrow Transplant. 2015;21(12):2039–2051. - PMC - PubMed
-
- Cook G, Ashcroft AJ, Cairns DA, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol. 2016;3(7):e340–e351. - PubMed
-
- Goldschmidt H, Baertsch M-A, Schlenzka J, et al. Salvage autologous transplant and lenalidomide maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: the randomized GMMG phase III trial ReLApsE. Leukemia. 2021;35(4):1134–1144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

