Enabling tumor-specific drug delivery by targeting the Warburg effect of cancer
- PMID: 39809265
- PMCID: PMC11866520
- DOI: 10.1016/j.xcrm.2024.101920
Enabling tumor-specific drug delivery by targeting the Warburg effect of cancer
Abstract
Metabolic reprogramming of tumor cells is an emerging hallmark of cancer. Among all the changes in cancer metabolism, increased glucose uptake and the accumulation of lactate under normoxic conditions (the "Warburg effect") is a common feature of cancer cells. In this study, we develop a lactate-responsive drug delivery platform by targeting the Warburg effect. We design and test a gold/mesoporous silica Janus nanoparticle system as a gated drug carrier, in which the gold particles are functionalized with lactate oxidase and the silica particles are capped with α-cyclodextrin through surface arylboronate modification. In the presence of lactate, the lactate oxidase generates hydrogen peroxide, which induces the self-immolation reaction of arylboronate, leading to uncapping and drug release. Our results demonstrate greatly improved drug delivery specificity and therapeutic efficacy with this platform for the treatment of different cancers. Our findings present an effective approach for drug delivery by metabolic targeting of tumors.
Keywords: Warburg effect; chemotherapy; drug delivery; immunotherapy; lactate; nanoparticle; tumor metabolism.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.Z. and X.W. are inventors for patent PCT/US2020/070052 (Lactate response system and methods). X.W. is a co-founder of Alnair Therapeutics, Inc.
Figures


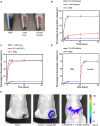
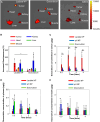
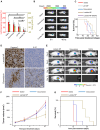



References
-
- Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013;12:991–1003. - PubMed
-
- Matsumoto Y., Nichols J.W., Toh K., Nomoto T., Cabral H., Miura Y., Christie R.J., Yamada N., Ogura T., Kano M.R., et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 2016;11:533–538. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

