A simple inland culture system provides insights into ascidian post-embryonic developmental physiology
- PMID: 39809318
- PMCID: PMC11732436
- DOI: 10.1098/rsob.240340
A simple inland culture system provides insights into ascidian post-embryonic developmental physiology
Abstract
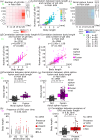
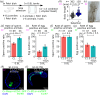
Maintenance and breeding of experimental organisms are fundamental to life sciences, but both initial and running costs, and hands-on zootechnical demands can be challenging for many laboratories. Here, we first aimed to further develop a simple protocol for reliable inland culture of tunicate model species of the Ciona genus. We cultured both Ciona robusta and Ciona intestinalis in controlled experimental conditions, with a focus on dietary variables, and quantified growth and maturation parameters. From statistical analysis of these standardized datasets, we gained insights into the post-embryonic developmental physiology of Ciona and inferred an improved diet and culturing conditions for sexual maturation. We showed that body length is a critical determinant of both somatic and sexual maturation, which suggests the existence of systemic control mechanisms of resource allocation towards somatic growth or maturation and supports applying size selection as a predictor of reproductive fitness in our inland culture to keep the healthiest animals at low density in the system. In the end, we successfully established a new protocol, including size selection, to promote both sperm and egg production. Our protocol using small tanks will empower researchers to initiate inland Ciona cultures with low costs and reduced space constraints.
Keywords: growth; growth burst; inland culture; maturation; tunicate.
Conflict of interest statement
We declare we have no competing interests.
Figures




Similar articles
-
Asymmetric Fitness of Second-Generation Interspecific Hybrids Between Ciona robusta and Ciona intestinalis.G3 (Bethesda). 2020 Aug 5;10(8):2697-2711. doi: 10.1534/g3.120.401427. G3 (Bethesda). 2020. PMID: 32518083 Free PMC article.
-
A study of the chorion and the follicle cells in relation to the sperm-egg interaction in the ascidian, Ciona intestinalis.Dev Biol. 1980 Feb;74(2):490-9. doi: 10.1016/0012-1606(80)90449-2. Dev Biol. 1980. PMID: 7371986 No abstract available.
-
Culture of Ciona intestinalis in closed systems.Dev Dyn. 2007 Jul;236(7):1832-40. doi: 10.1002/dvdy.21124. Dev Dyn. 2007. PMID: 17394236
-
Ciona intestinalis and Oxycomanthus japonicus, representatives of marine invertebrates.Exp Anim. 2009 Oct;58(5):459-69. doi: 10.1538/expanim.58.459. Exp Anim. 2009. PMID: 19897929 Review.
-
Ion currents involved in oocyte maturation, fertilization and early developmental stages of the ascidian Ciona intestinalis.Mol Reprod Dev. 2011 Oct-Nov;78(10-11):854-60. doi: 10.1002/mrd.21316. Epub 2011 Apr 25. Mol Reprod Dev. 2011. PMID: 21520326 Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

