Intra-arterial tenecteplase after successful endovascular recanalisation in patients with acute posterior circulation arterial occlusion (ATTENTION-IA): multicentre randomised controlled trial
- PMID: 39809509
- PMCID: PMC11729139
- DOI: 10.1136/bmj-2024-080489
Intra-arterial tenecteplase after successful endovascular recanalisation in patients with acute posterior circulation arterial occlusion (ATTENTION-IA): multicentre randomised controlled trial
Abstract
Objective: To assess whether intra-arterial tenecteplase administered after successful endovascular recanalisation improves outcomes in patients with acute arterial occlusion of the posterior circulation.
Design: Multicentre randomised controlled trial.
Setting: 31 hospitals in China, 24 January 2023 to 24 August 2023.
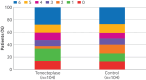
Participants: 208 patients with successful recanalisation (grade 2b50-3 on the extended thrombolysis in cerebral infarction scale) of an occlusion in the V4 segment of the vertebral artery; proximal, middle, or distal segment of the basilar artery; or P1 segment of the posterior cerebral artery: 104 were randomly allocated to receive tenecteplase and 104 to receive standard care.
Interventions: Intra-arterial tenecteplase (0.0625 mg/kg, maximum dose 6.25 mg) administered proximal to the residual thrombus (if still present) or distal to the origin of the main pontine perforator branches over 15 seconds, or endovascular treatment only (control group).
Main outcome measures: The primary outcome was freedom from disability (modified Rankin scale score 0 or 1) at 90 days after randomisation. Primary safety outcomes included symptomatic intracranial haemorrhage within 36 hours and all cause mortality at 90 days. All efficacy and safety analyses were conducted by intention to treat and adjusted for age, pre-stroke modified Rankin scale score, time from onset of moderate to severe stroke (National Institutes of Health stroke scale score ≥6) to randomisation, hypertension, and baseline stroke severity.
Results: At 90 days, 36 patients (34.6%) in the tenecteplase group and 27 (26.0%) in the control group had a modified Rankin scale score of 0 or 1 (adjusted risk ratio 1.36, 95% confidence interval 0.92 to 2.02; P=0.12). Mortality at 90 days was similar between the tenecteplase and control groups: 29 (27.9%) v 28 (26.9%), adjusted risk ratio 1.13, 0.73 to 1.74. Symptomatic intracranial haemorrhage within 36 hours occurred in eight patients (8.3%) in the tenecteplase group and three (3.1%) in the control group (adjusted risk ratio 3.09, 0.78 to 12.20).
Conclusions: In patients with acute ischaemic stroke due to acute posterior large or proximal vessel occlusion, intra-arterial tenecteplase administered after successful recanalisation was not associated with a statistically significant reduction in combined disability and mortality at 90 days.
Trial registration: ClinicalTrials.gov NCT05684172.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: funding from the Fundamental Research Funds for the Central Universities; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Tao C, Qureshi AI, Yin Y, et al. ATTENTION (Endovascular Treatment for Acute Basilar Artery Occlusion) Trial Investigators . Endovascular Treatment Versus Best Medical Management in Acute Basilar Artery Occlusion Strokes: Results From the ATTENTION Multicenter Registry. Circulation 2022;146:6-17. 10.1161/CIRCULATIONAHA.121.058544 - DOI - PubMed
-
- National Clinical Guideline for Stroke for the UK and Ireland. London: Intercollegiate Stroke Working Party; 2023 May 4. www.strokeguideline.org.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
