Disparities in dolutegravir utilisation in children, adolescents and young adults (0-24 years) living with HIV. An analysis of the IeDEA Pediatric West African cohort
- PMID: 39809526
- PMCID: PMC11749752
- DOI: 10.1136/bmjgh-2024-016512
Disparities in dolutegravir utilisation in children, adolescents and young adults (0-24 years) living with HIV. An analysis of the IeDEA Pediatric West African cohort
Abstract
Introduction: We describe the 24-month incidence of Dolutegravir (DTG)-containing antiretroviral treatment (ART) initiation since its introduction in 2019 in West Africa.
Methods: We included all patients aged 0-24 years on ART from nine clinics in Côte d'Ivoire (n=4), Ghana, Nigeria, Mali, Benin, and Burkina Faso. Baseline varied by clinic and was defined as date of first DTG prescription; patients were followed up until database closure/death/loss to follow-up (LTFU, no visit ≥7 months), whichever came first. We computed the cumulative incidence function for DTG initiation; associated factors were explored in a shared frailty model, accounting for clinic heterogeneity.
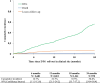
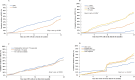
Results: Since 2019, 3350 patients were included; 47.2% were female; 78.9% had been on ART ≥12 months. Median baseline age was 12.5 years (IQR 8.4-15.8). Median follow-up was 14 months (IQR 7-22). The overall cumulative incidence of DTG initiation reached 22.7% (95% CI 21.3 to 24.2) and 56.4% (95% CI 54.4 to 58.4) at 12 and 24 months, respectively. In univariate analyses, those aged <5 years and female were overall less likely to switch. Adjusted on ART line and available viral load (VL) at baseline, females aged >10 years were less likely to initiate DTG compared with males of the same age (adjusted HR among 10-14 years: 0.62, 95% CI 0.54 to 0.72; among ≥15 years: 0.43, 95% CI 0.36 to 0.50), as were those with detectable VL (>50 copies/mL) compared with those in viral suppression (aHR 0.86, 95% CI 0.77 to 0.97) and those on PIs compared with those on non-nucleoside reverse-transcriptase inhibitors (aHR after 12 months of roll-out: 0.75, 95% CI 0.65 to 0.86).
Conclusion: Paediatric DTG uptake was incomplete and unequitable in west African settings: DTG use was least likely in children <5 years, females ≥10 years and those with detectable VL. Maintained monitoring and support of treatment practices is required to better ensure universal and equal uptake.
Keywords: Cohort study; HIV; Paediatrics; Public Health.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

Update of
-
Disparities in dolutegravir utilisation in children, adolescents and young adults (0-24 years) living with HIV: An analysis of the IeDEA Paediatric West African cohort.medRxiv [Preprint]. 2024 Nov 8:2024.05.24.24307900. doi: 10.1101/2024.05.24.24307900. medRxiv. 2024. Update in: BMJ Glob Health. 2025 Jan 14;10(1):e016512. doi: 10.1136/bmjgh-2024-016512. PMID: 38826257 Free PMC article. Updated. Preprint.
References
-
- Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd. Geneva: World Health Organization; 2016. edn. - PubMed
-
- World Health Organisation Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. 2018
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
