Infiltrating peripheral monocyte TREM-1 mediates dopaminergic neuron injury in substantia nigra of Parkinson's disease model mice
- PMID: 39809747
- PMCID: PMC11733277
- DOI: 10.1038/s41419-025-07333-5
Infiltrating peripheral monocyte TREM-1 mediates dopaminergic neuron injury in substantia nigra of Parkinson's disease model mice
Abstract
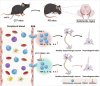
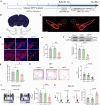
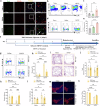
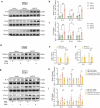
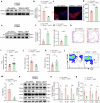
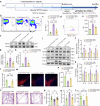
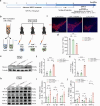
Neuroinflammation is a key factor in the pathogenesis of Parkinson's disease (PD). Activated microglia in the central nervous system (CNS) and infiltration of peripheral immune cells contribute to dopaminergic neuron loss. However, the role of peripheral immune responses, particularly triggering receptor expressed on myeloid cells-1 (TREM-1), in PD remains unclear. Using a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP)-induced PD mouse model, we examined TREM-1 expression and monocyte infiltration in the substantia nigra pars compacta (SNpc). We found that MPTP increased peripheral monocytes, and deletion of peripheral monocytes protected against MPTP neurotoxicity in the SNpc. TREM-1 inhibition, both genetically and pharmacologically, reduced monocyte infiltration, alleviated neuroinflammation, and preserved dopaminergic neurons, resulting in improved motor function. Furthermore, adoptive transfer of TREM-1-expressing monocytes from PD model mice to naive mice induced neuronal damage and motor deficits. These results underscore the critical role of peripheral monocytes and TREM-1 in PD progression, suggesting that targeting TREM-1 could be a promising therapeutic approach to prevent dopaminergic neurodegeneration and motor dysfunction in PD. Schematic diagram of monocyte TREM-1-mediated dopaminergic neuron damage. The figure illustrates that in experimental MPTP-induced PD model mice, the number of inflammatory monocytes in the peripheral blood increases, after which the monocytes infiltrate the CNS through the Blood-Brain Barrier(BBB). These infiltrating monocytes increase the release of inflammatory cytokines and eventually cause neuronal injury. TREM-1 gene deletion and pharmacological blockade limit inflammatory monocyte recruitment into the SNpc and ameliorate neuroinflammatory events and the loss of dopaminergic neurons.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Consent for publication: All the authors read and approved the publication of this manuscript. Ethics approval and consent to participate: Animal protocols were approved by the Institutional Animal Care and Use Committee of Xuzhou Medical University.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

