Transarterial Radioembolisation with Y90 Resin Microspheres and the Effect of Reimbursement Criteria in France: Final Results of the CIRT-FR Prospective Observational Study
- PMID: 39809885
- PMCID: PMC11790776
- DOI: 10.1007/s00270-024-03955-y
Transarterial Radioembolisation with Y90 Resin Microspheres and the Effect of Reimbursement Criteria in France: Final Results of the CIRT-FR Prospective Observational Study
Abstract
Purpose: This analysis of the CIRSE Registry for SIR-Spheres Therapy in France, CIRT-FR, reports on real-world outcomes of transarterial radioembolisation (TARE) with Y90 resin microspheres for hepatocellular carcinoma (HCC) and colorectal cancer liver metastases (CRLM) patients in France, focusing on safety, effectiveness and health-related quality of life (HRQoL). Results on patients treated based on national reimbursement criteria are discussed here.
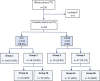
Methods: Prospective, multicentre, observational study of HCC and CRLM patients treated between August 2017 and July 2020 with TARE Y90 resin microspheres. Patients were assigned to different analysis groups based on reimbursement recommendations. Follow-up period was at least 24 months with patient data collected every 3 months.
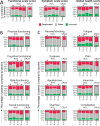
Results: In total, 252 (193 HCC, 59 CRLM) patients of CIRT-FR were included in the analysis. No differences in effectiveness, safety and HRQoL were found between analysis groups based on reimbursement recommendations. Median overall survival for HCC and CRLM was 19.0 (95% CI, 16.1-22.4) and 10.8 (95% CI, 8.0-13.5) months, respectively. Serious procedure-related adverse events occurred in 13% of the patients. HRQoL generally remained stable, with some fluctuations in function scores and symptoms.
Conclusion: In our cohorts, patients performed similarly regarding clinical outcomes irrespective of their analysis group based on reimbursement recommendations. Our results suggest that instead of restrictive reimbursement criteria, more decision-making power in selecting suitable patient groups could be given to multidisciplinary tumour boards. Results confirm that TARE with Y90 resin microspheres is an effective and safe treatment for liver cancer, with maintained HRQoL in most patients.
Keywords: Colorectal liver metastases; Hepatocellular carcinoma; Liver; Reimbursement; Resin microspheres; Transarterial radioembolisation; Yttrium-90.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Valérie Vilgrain received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, and payment for expert testimony from Sirtex. Dirk Arnold received consulting fees and honoraria for presentations and lectures and travel support from Boston Scientific and Terumo, MSD, BMS, AstraZeneca, Roche, Servier, Sanofi and Merck Serono. He is on the guidelines committee of the European Society for Medical Oncology and supported oncopolicy manuscripts for the European Cancer Organisation. Maxime Ronot received honoraria for lectures from GE Healthcare, Ipsen, Canon-Toshiba, Alexion Pharmaceuticals, Guerbet, and Sirtex. Geert Maleux received honoraria for speaker’s bureau from Sirtex Medical and operated as a proctor for Sirtex. Bruno Sangro received grants or contracts from Sirtex and BMS, consulting fees from Adaptimmune, Astra Zeneca, Bayer, BMS, Boston Scientific, Eisai, Eli Lilly, Incyte, Ipsen, Roche, Sirtex Medical, Terumo; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astra Zeneca, Bayer, BMS, Eisai, Incyte, Ipsen, Roche, Sirtex Medical; Participation on a Data Safety Monitoring Board or Advisory Board from Adaptimmune, Astra Zeneca, Bayer, BMS, Boston Scientific, Eisai, Eli Lilly, Incyte, Ipsen, Roche, Sirtex Medical, Terumo, and has a leadership or fiduciary role in the International Liver Cancer Association. Michel Greget recieved honoraria from Sirtex as a lecturer in educationnal events and operated as a proctor for Sirtex. Romaric Loffroy received honoraria for lectures from Sirtex. Olivier Pellerin received honoraria for lectures from Merit Medical and he is co-founder of PERSEA. Thomas Helmberger, Christian Sengel, Niklaus Schaefer, Bora Peynircioglu, Maria Urdaniz, Bleranda Zeka and Niels de Jong had nothing to declare. Ethical Approval: All procedures performed in CIRT-FR were in accordance with the ethical standards of the national ethics committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study has been approved by the applicable ethics committee. Informed Consent: All participants signed an informed consent form.
Figures

References
-
- Garin E, Rolland Y, Edeline J, et al. Personalized dosimetry with intensification using90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J Nucl Med. 2015;56:339–46. 10.2967/jnumed.114.145177. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

