In vivo base editing extends lifespan of a humanized mouse model of prion disease
- PMID: 39810005
- PMCID: PMC12003183
- DOI: 10.1038/s41591-024-03466-w
In vivo base editing extends lifespan of a humanized mouse model of prion disease
Erratum in
-
Author Correction: In vivo base editing extends lifespan of a humanized mouse model of prion disease.Nat Med. 2025 Apr;31(4):1369. doi: 10.1038/s41591-025-03540-x. Nat Med. 2025. PMID: 39885360 Free PMC article. No abstract available.
Abstract
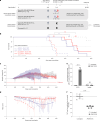
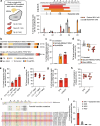
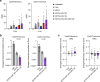
Prion disease is a fatal neurodegenerative disease caused by the misfolding of prion protein (PrP) encoded by the PRNP gene. While there is currently no cure for the disease, depleting PrP in the brain is an established strategy to prevent or stall templated misfolding of PrP. Here we developed in vivo cytosine and adenine base strategies delivered by adeno-associated viruses to permanently modify the PRNP locus to achieve PrP knockdown in the mouse brain. Systemic injection of dual-adeno-associated virus PHP.eB encoding BE3.9max and single guide RNA installing PRNP R37X resulted in 37% average installation of the desired edit, 50% reduction of PrP in the mouse brain and 52% extension of lifespan in transgenic human PRNP mice inoculated with pathogenic human prion isolates representing the most common sporadic and genetic subtypes of prion disease. We further engineered base editing systems to achieve improved in vivo potency and reduced base editor expression in nontargeting tissues, resulting in 63% average PrP reduction in the mouse brain from a 6.7-fold lower viral dose, with no detected off-target editing of anticipated clinical significance observed in either human cells or mouse tissues. These findings support the potential of in vivo base editing as one-time treatment for prion disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.A., J.R.D., E.V.M., S.M.V. and D.R.L. are inventors on United States patent applications 63/700,235 and 63/718,534 relating to base editing for prion disease. D.R.L. is a consultant and/or equity owner of Prime Medicine, Beam Therapeutics, Pairwise Plants, Exo Therapeutics, Nvelop Therapeutics and Chroma Medicine, some of which are companies that use or deliver genome editing or epigenome-modulating agents. J.R.D., J.M.L. and W.-H.Y. are current employees of Prime Medicine. S.M.V. acknowledges speaking fees from Abbvie, Biogen, Eli Lilly, Illumina and Ultragenyx; consulting fees from Alnylam and Invitae; and research support from Eli Lilly, Gate Bio, Ionis and Sangamo. E.V.M. acknowledges speaking fees from Abbvie, Eli Lilly and Vertex; consulting fees from Alnylam and Deerfield; and research support from Eli Lilly, Gate Bio, Ionis and Sangamo. B.E.D. declares outside interest in Apertura Gene Therapy and Tevard Biosciences and is an inventor on US patent application US11499165B2 relating to the PHP.eB AAV capsid. All other authors declare no competing interests.
Figures







References
-
- Bueler, H. et al. Mice devoid of PrP are resistant to scrapie. Cell73, 1339–1347 (1993). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

