Targeting of the G9a, DNMT1 and UHRF1 epigenetic complex as an effective strategy against pancreatic ductal adenocarcinoma
- PMID: 39810240
- PMCID: PMC11734372
- DOI: 10.1186/s13046-024-03268-5
Targeting of the G9a, DNMT1 and UHRF1 epigenetic complex as an effective strategy against pancreatic ductal adenocarcinoma
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer with limited treatment options and a poor prognosis. The critical role of epigenetic alterations such as changes in DNA methylation, histones modifications, and chromatin remodeling, in pancreatic tumors progression is becoming increasingly recognized. Moreover, in PDAC these aberrant epigenetic mechanisms can also limit therapy efficacy. This study aimed to investigate the expression and prognostic significance of a key epigenetic complex encompassing DNA methyltransferase-1 (DNMT1), the histone methyltransferase G9a, and the scaffold protein UHRF1 in PDAC. We also evaluated the therapeutic potential of an innovative inhibitor targeting these epigenetic effectors.
Methods: Immunohistochemical analysis of DNMT1, G9a, and UHRF1 expression was conducted in human PDAC tissue samples. Staining was semi-quantitatively scored, and overexpression was defined as moderate to strong positivity. The prognostic impact was assessed by correlating protein expression with patient survival. The antitumoral effects of the dual DNMT1-G9a inhibitor CM272 were tested in PDAC cell lines, followed by transcriptomic analyses to identify underlying mechanisms. The in vivo antitumoral efficacy of CM272 was evaluated in PDAC xenograft and syngeneic mouse models, both alone and in combination with anti-PD1 immunotherapy.
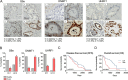
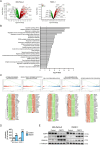
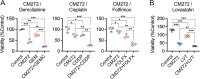
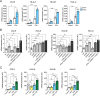
Results: DNMT1, G9a, and UHRF1 were significantly overexpressed in PDAC cells and stroma compared to normal pancreatic tissues. Simultaneous overexpression of the three proteins was associated with significantly reduced survival in resected PDAC patients. CM272 exhibited potent antiproliferative activity in PDAC cell lines, inducing apoptosis and altering key metabolic and cell cycle-related genes. CM272 also enhanced chemotherapy sensitivity and significantly inhibited tumor growth in vivo without detectable toxicity. Combination of CM272 with anti-PD1 therapy further improved antitumor responses and immune cell infiltration, particularly CD4 + and CD8 + T cells.
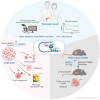
Conclusions: The combined overexpression of DNMT1, G9a, and UHRF1 in PDAC is a strong predictor of poor prognosis. CM272, by targeting this epigenetic complex, shows promising therapeutic potential by inducing apoptosis, reprogramming metabolic pathways, and enhancing immune responses. The combination of CM272 with immunotherapy offers a novel, effective treatment strategy for PDAC.
Keywords: Apoptosis; CM272; Chemotherapy sensitivity; DNMT1; Epigenetic mechanisms; G9a; Immunotherapy; Metabolic reprogramming; Pancreatic ductal adenocarcinoma; UHRF1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The collection of PDAC patient samples was performed at two different research institutions while adhering to ethical guidelines and informed consent procedures. The study protocol was reviewed and approved by the local Ethical Committee of the University Hospital of Modena (Comitato Etico dell'Area Vasta Emilia Nord- Protocol Number: 0013043/19—Pratica 299/2019/OSS/AOUMO) and the Clinical Research Ethical Committee of Navarra (Pyto2015/120). All patients provided written informed consent. All animal procedures involving mice were performed in compliance with regulations concerning animals used in scientific research. The animal procedures in the VHIO were approved by the institution's Ethical Committee and the Catalan Regional Government., while the CIMA-University of Navarra approved the project under project approval #R-CP001-15GN. Consent for publication: All authors read this manuscript and approve for publication. Competing interests: The authors declare that they have no competing interest.
Figures



References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. 10.3322/CAAC.21820. - PubMed
-
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–20. 10.1016/S0140-6736(20)30974-0. - PubMed
-
- Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17:108–23. 10.1038/S41571-019-0281-6. - PubMed
-
- Yu KH. Advances in Systemic Therapy in Pancreatic Cancer. Hematol Oncol Clin North Am. 2024;38:617–27. 10.1016/J.HOC.2024.03.002. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

