Local Inflammation Precedes Diaphragm Wasting and Fibrotic Remodelling in a Mouse Model of Pancreatic Cancer
- PMID: 39810606
- PMCID: PMC11733308
- DOI: 10.1002/jcsm.13668
Local Inflammation Precedes Diaphragm Wasting and Fibrotic Remodelling in a Mouse Model of Pancreatic Cancer
Abstract
Background: Cancer cachexia represents a debilitating muscle wasting condition that is highly prevalent in gastrointestinal cancers, including pancreatic ductal adenocarcinoma (PDAC). Cachexia is estimated to contribute to ~30% of cancer-related deaths, with deterioration of respiratory muscles suspected to be a key contributor to cachexia-associated morbidity and mortality. In recent studies, we identified fibrotic remodelling of respiratory accessory muscles as a key feature of human PDAC cachexia.
Methods: To gain insight into mechanisms driving respiratory muscle wasting and fibrotic remodelling in response to PDAC, we conducted temporal histological and transcriptomic analyses on diaphragm muscles harvested from mice-bearing orthotopic murine pancreatic (KPC) tumours at time points reflective of precachexia (D8 and D10), mild-moderate cachexia (D12 and D14) and advanced cachexia (endpoint).
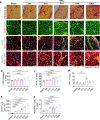
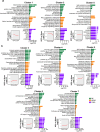
Results: During the precachexia phase, diaphragms showed significant leukocyte infiltration (+3-fold to +13-fold; D8-endpoint vs. Sham, p < 0.05) and transcriptomic enrichment of inflammatory processes associated with tissue injury that remained increased through endpoint. Diaphragm inflammation was followed by increases in PDGFR-ɑ+ fibroadipogenic progenitors (+2.5 to +3.8-fold; D10-endpoint vs. Sham, p < 0.05), fibre atrophy (-16% to -24%, D12 to endpoint vs. Sham, p < 0.05), ECM expansion (+1.5 to +1.8-fold; D14-endpoint vs. Sham, p < 0.05), collagen accumulation (+3.8-fold; endpoint vs. Sham, p = 0.0013) and reductions in breathing frequency (-55%, p = 0.0074) and diaphragm excursion (-43%, p = 0.0006). These biological processes were supported by changes in the diaphragm transcriptome. Ingenuity pathway analysis predicted factors involved in inflammatory responses to tissue injury, including TGF-β1, angiotensin and PDGF BB, as top upstream regulators activated in diaphragms prior to and throughout cachexia progression, while PGC-1α and the insulin receptor were among the top upstream regulators predicted to be suppressed. The transcriptomic dataset further revealed progressive disturbances to networks involved in lipid, glucose and oxidative metabolism, activation of the unfolded protein response and neuromuscular junction remodelling associated with denervation.
Conclusions: In summary, our data support leukocyte infiltration and expansion of PDGFRα mesenchymal progenitors as early events that precede wasting and fibrotic remodelling of the diaphragm in response to PDAC that may also underlie metabolic disturbances, weakness and respiratory complications.
Keywords: cancer cachexia; inflammatory response; muscle atrophy; muscle fibrosis; pancreatic cancer.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Fearon K., Arends J., and Baracos V., “Understanding the Mechanisms and Treatment Options in Cancer Cachexia,” Nature Reviews Clinical Oncology 10 (2013): 90–99. - PubMed
-
- Bachmann J., Heiligensetzer M., Krakowski‐Roosen H., Buchler M. W., Friess H., and Martignoni M. E., “Cachexia Worsens Prognosis in Patients With Resectable Pancreatic Cancer,” Journal of Gastrointestinal Surgery 12 (2008): 1193–1201. - PubMed
-
- Argiles J. M., Stemmler B., Lopez‐Soriano F. J., and Busquets S., “Inter‐Tissue Communication in Cancer Cachexia,” Nature Reviews. Endocrinology 15 (2018): 9–20. - PubMed
-
- Baracos V. E., Martin L., Korc M., Guttridge D. C., and Fearon K. C. H., “Cancer‐Associated Cachexia,” Nature Reviews Disease Primers 4 (2018): 17105. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

