Efficacy and Safety of Pegcetacoplan in Kidney Transplant Recipients With Recurrent Complement 3 Glomerulopathy or Primary Immune Complex Membranoproliferative Glomerulonephritis
- PMID: 39810766
- PMCID: PMC11725963
- DOI: 10.1016/j.ekir.2024.09.030
Efficacy and Safety of Pegcetacoplan in Kidney Transplant Recipients With Recurrent Complement 3 Glomerulopathy or Primary Immune Complex Membranoproliferative Glomerulonephritis
Abstract
Introduction: Complement 3 glomerulopathy (C3G) and primary immune complex membranoproliferative glomerulonephritis (IC-MPGN) have high risks for disease recurrence and allograft loss in transplant kidneys. Pegcetacoplan (targeted complement 3 [C3]/C3b inhibitor) may prevent excessive deposition of C3 and complement 5 [C5] breakdown products and associated renal damage.
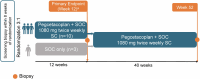
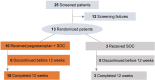
Methods: NOBLE (NCT04572854) is a prospective, phase 2, multicenter, open-label, randomized controlled trial evaluating the efficacy and safety of pegcetacoplan in posttransplant patients with recurrent C3G or IC-MPGN. The primary end point was reduction in C3c staining on renal biopsy at week 12 for patients who received either pegcetacoplan 1080 mg twice weekly by subcutaneous infusion plus standard-of-care (SOC) or SOC only.
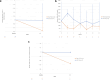
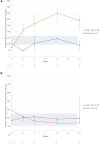
Results: Ten patients received pegcetacoplan and 3 received SOC only through week 12. At week 12, 5 of 10 pegcetacoplan-treated patients (50%) achieved ≥2 orders of magnitude (OOM) reduction in C3 staining (4 of these 5 had 0 staining and absent electron microscopy deposits) and 8 of 10 (80%) achieved ≥1 OOM reduction; 1 of 3 (33%) SOC-only patients showed staining reduction. Mean C3G histology activity score decreased by >54% in 8 of 10 pegcetacoplan-treated patients (80.0%). Pegcetacoplan-treated patients with baseline urine protein-to-creatinine ratio (uPCR) ≥1000 mg/g showed a median (interquartile range [IQR]) 54.4% (-56.33 to -53.95) reduction in proteinuria at week 12. In addition, pegcetacoplan-treated patients showed stable estimated glomerular filtration rate (eGFR), reduced plasma sC5b-9, and increased serum C3. Pegcetacoplan was well-tolerated and most adverse events were mild/moderate. No discontinuations, treatment withdrawals, or deaths were reported.
Conclusion: NOBLE demonstrated efficacy, safety, and tolerability of pegcetacoplan for patients with posttransplant recurrent C3G and primary IC-MPGN.
Keywords: C3 glomerulopathy; complement; immune complex membranoproliferative glomerulonephritis; pegcetacoplan; proteinuria.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures


Comment in
-
Anticomplement Therapies for C3 Glomerulopathy and Immune-Complex Membranoproliferative Glomerulonephritis Recurrence - A Dawn of New Hope.Kidney Int Rep. 2024 Nov 19;10(1):7-9. doi: 10.1016/j.ekir.2024.11.018. eCollection 2025 Jan. Kidney Int Rep. 2024. PMID: 39812304 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

