Shaping Treatment Expectation to Optimize Efficacy of Interleukin 17A Antagonist Secukinumab in Psoriasis Patients
- PMID: 39810930
- PMCID: PMC11731016
- DOI: 10.2147/PTT.S486338
Shaping Treatment Expectation to Optimize Efficacy of Interleukin 17A Antagonist Secukinumab in Psoriasis Patients
Abstract
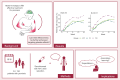
Purpose: Patients' treatment expectations significantly influence the effectiveness of medical and pharmacological treatments. This clinical proof-of-concept study aimed to enhance treatment outcomes by targeting positive treatment expectations of psoriasis patients beginning systemic anti-psoriatic therapy with secukinumab, an interleukin (IL)-17A antagonist.
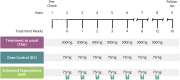
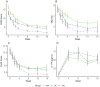
Patients and methods: We randomly assigned patients to three groups: a treatment as usual (TAU) group receiving the standard 300mg dose of secukinumab, a dose-control (DC) group with 75% dose reduction and an experimental (EXP) group receiving the same reduced dose along with a "cover story" designed to positively influence treatment expectations. We evaluated skin symptoms using the Psoriasis Area and Severity Index (PASI), the Dermatology Life Quality Index (DLQI), perceived itch, mood and plasma IL-17A levels at baseline and at 1, 2, 3, 4, 8, 12, and 16 weeks post intervention.
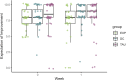
Results: The study included N = 120 patients (average age = 45.78 years, 34% female). A high baseline expectation level (8.1 of 10 points) was observed across all groups which could not be further increased by the EXP-group's "cover story". The EXP and DC groups did not differ with regard to reaching 75% improvement in PASI scores (PASI75), a DLQI score of 0 or 1 or at least 4 points improvement in itch. Over time, the EXP-group showed a faster decline in PASI scores and anxiety symptoms compared to the DC-group, but less improvement in quality of life. IL-17A levels significantly increased throughout the treatment, with no significant differences between groups despite the 75% dose reduction.
Conclusion: This study demonstrates an attempt to modify patients' treatment expectations to enhance the effectiveness of pharmacological therapy with secukinumab in psoriasis patients. However, verbal suggestion alone did not significantly improve clinical outcomes, suggesting that future studies should explore alternative approaches to leverage placebo effects to the benefit of patients with psoriasis.
Keywords: placebo effect; psoriasis treatment; psychodermatology.
© 2025 Hölsken et al.
Conflict of interest statement
SH, SM and DB report no conflict of interest. Frederik Krefting received travel support for participation in congresses and / or (speaker) honoraria from Novartis, Lilly, Bristol-Myers Squibb, Janssen, Almirall, and Boehringer Ingelheim outside of the present publication. MS reports speaker honoraria from Lilly, Allergopharma, NovoNordisk, Sanofi, Janssen, InVo, Bristol-Meyers, NCO, Almirall, and Berlin Chemie. WS reports grants and/or travel support and/or personal fees and/or speaker honoraria from medi GmbH Bayreuth, AbbVie, Almirall, Amgen, Bristol-Myers Squibb, Celgene, GSK, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi Genzyme, and UCB outside the submitted work. The authors report no other conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

