Thermal Behavior of n-Octanol and Related Ether Alcohols
- PMID: 39811100
- PMCID: PMC11726550
- DOI: 10.1021/acs.jced.4c00525
Thermal Behavior of n-Octanol and Related Ether Alcohols
Abstract
The thermal behavior of n-octanol and related ether alcohols has been studied by differential scanning calorimetry (DSC). The melting point, heat of fusion, and isobaric heat capacities of n-octanol obtained from the DSC measurements are in good agreement with literature values. The ether alcohols display kinetic barriers for forming a solid phase during cooldown. These barriers are least for 6-methoxyhexanol that forms a solid upon cooling except for the highest measured temperature change rate of 40 K·min-1, followed by 4-propoxybutanol that forms a solid during cooldown only at low cooling rates. 2-Pentoxyethanol and 5-ethoxypentanol form a solid during the heating cycle that then melts again upon further heating. 3-Butoxypropanol does not display any exo- and endothermic features for all measured temperature change rates. Consequently, new data on melting point and heats of fusion are reported for the ether alcohols except for 3-butoxypropanol. New isobaric heat capacities are presented as well for the liquid phase of these ether alcohols.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

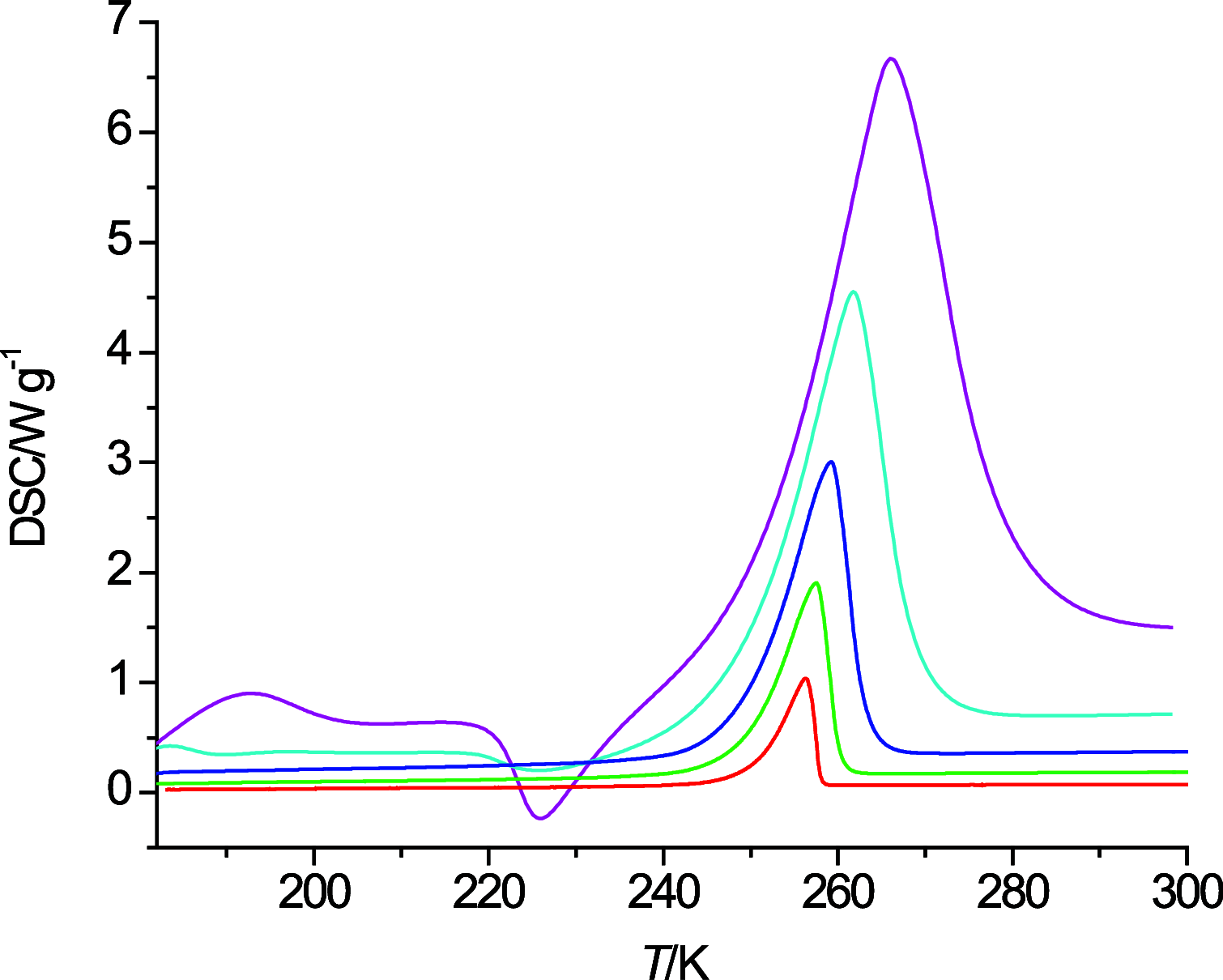
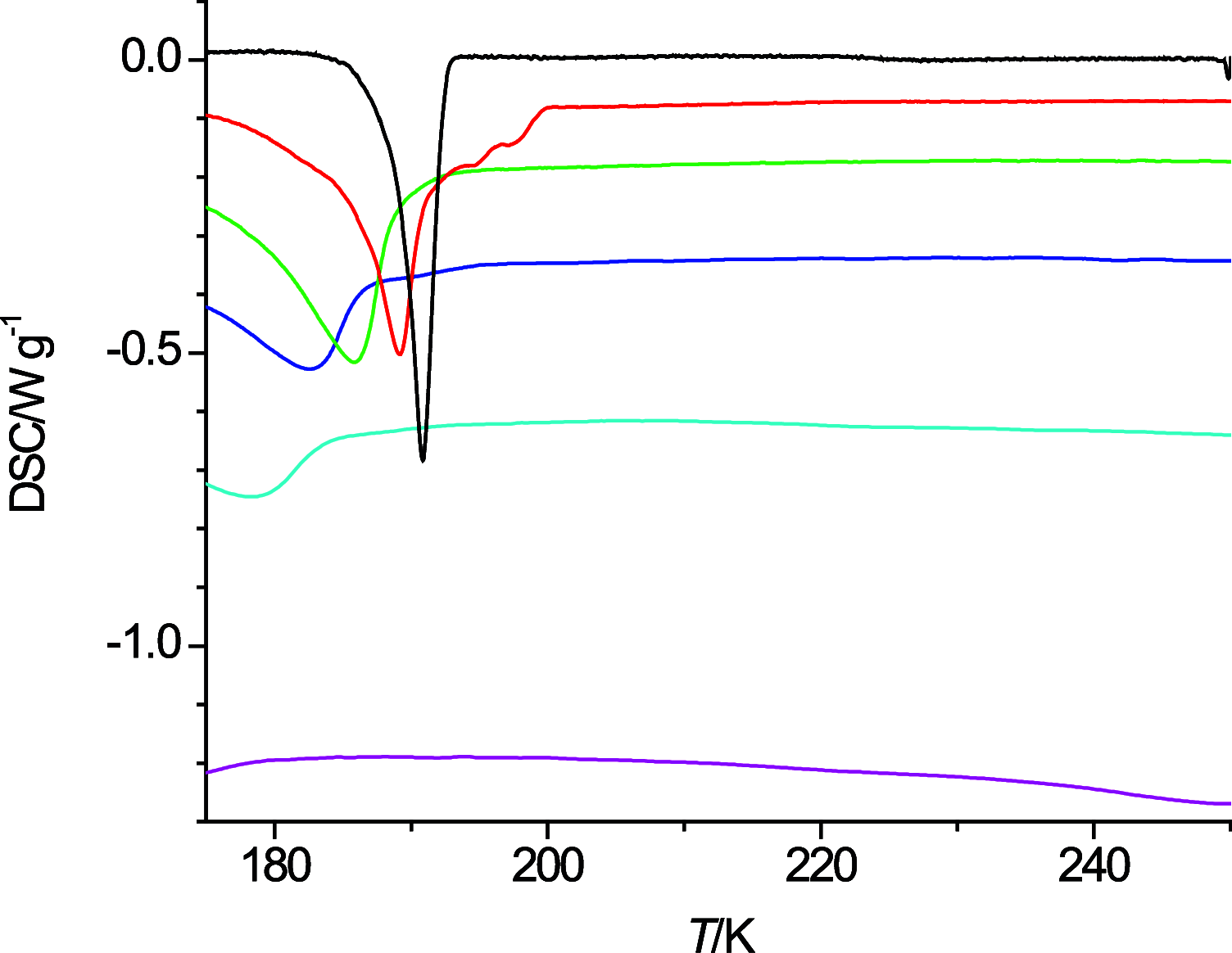


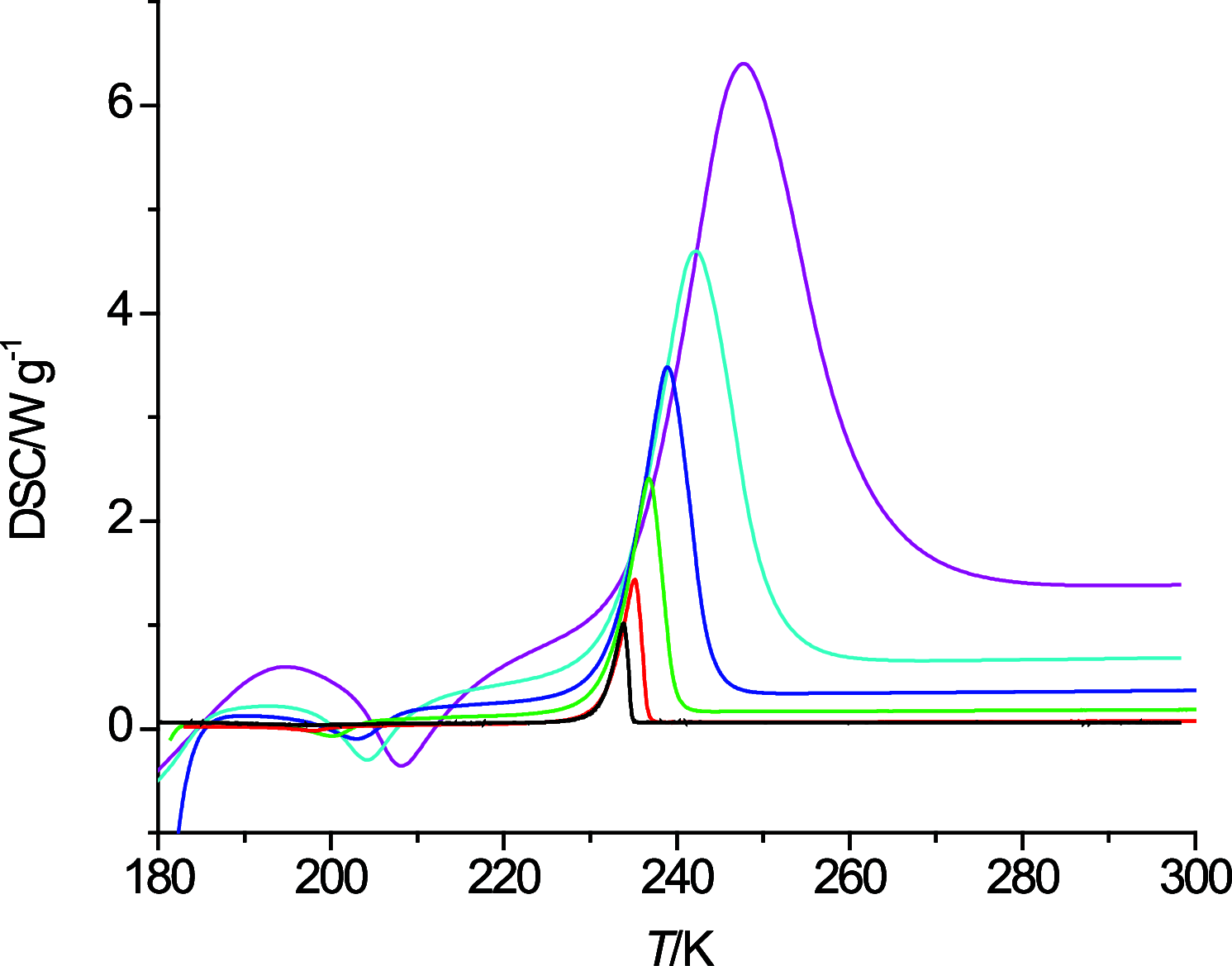
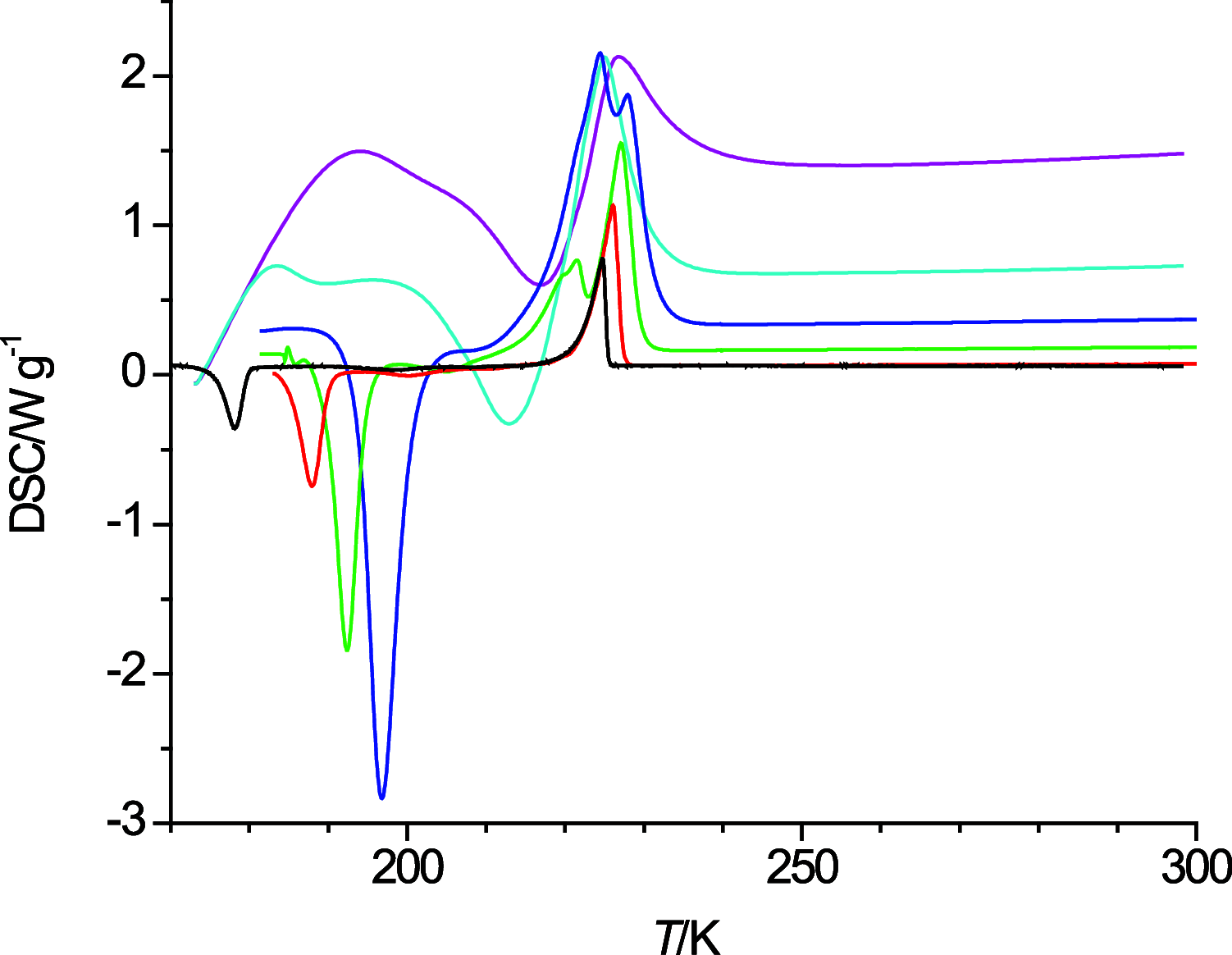
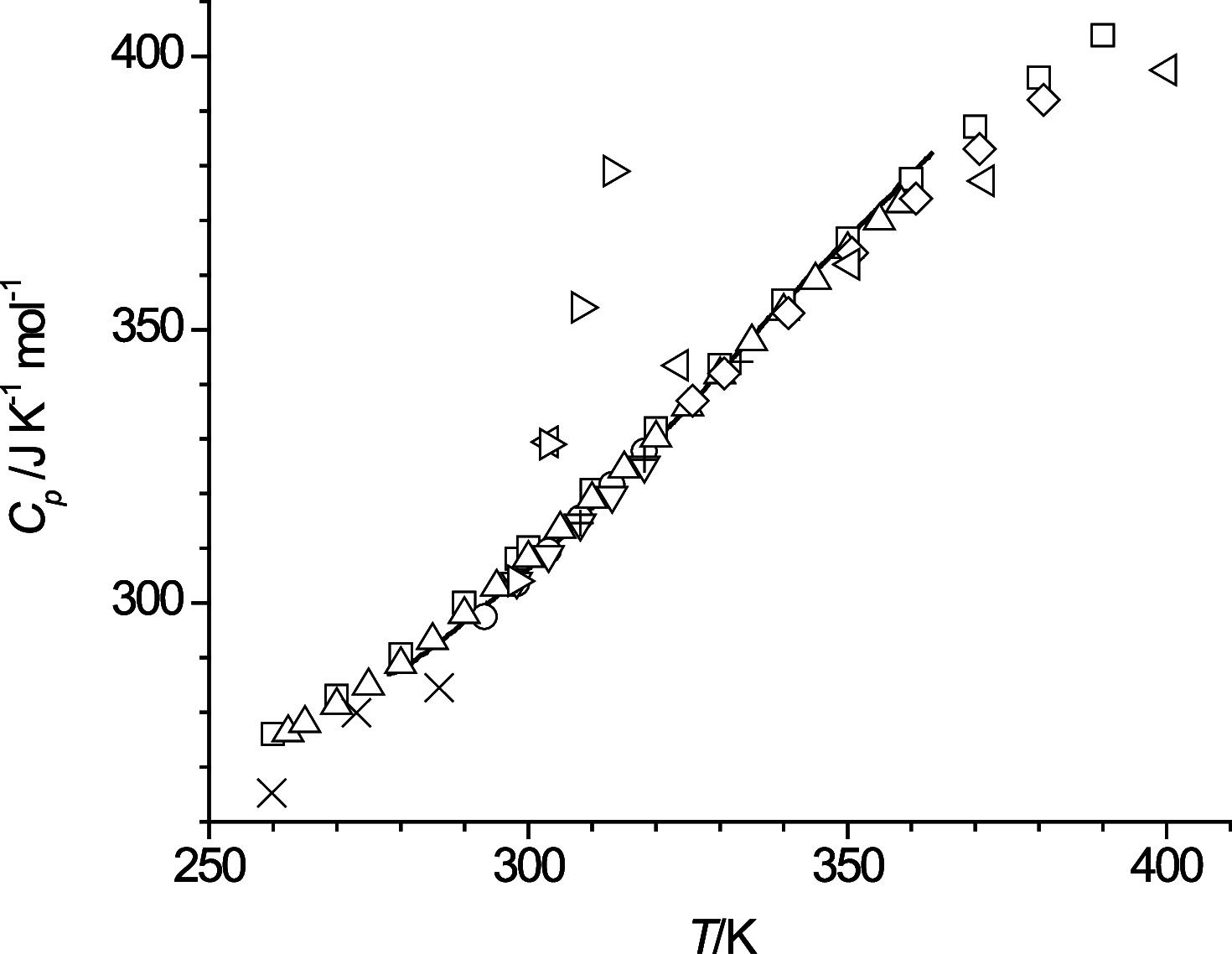
References
-
- Kardooni R.; Kiasat A. R. Polyethylene Glycol as a Green and Biocompatible Reaction Media for the Catalyst Free Synthesis of Organic Compounds. Curr. Org. Chem. 2020, 24, 1275–1314. 10.2174/1385272824999200605161840. - DOI
-
- Campos J. F.; Berteina-Raboin S. Greener Synthesis of Nitrogen-Containing Heterocycles in Water, PEG, and Bio-Based Solvents. Catalysts 2020, 10, 429.10.3390/catal10040429. - DOI
-
- Soni J.; Sahiba N.; Sethiya A.; Agarwal S. Polyethylene Glycol: A Promising Approach for Sustainable Organic Synthesis. J. Mol. Liq. 2020, 315, 113766.10.1016/j.molliq.2020.113766. - DOI
-
- Hoffmann M. M. Polyethylene Glycol as a Green Chemical Solvent. Curr. Opin. Colloid Interface Sci. 2022, 57, 101537.10.1016/j.cocis.2021.101537. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
