Homologous-adhering/targeting cell membrane- and cell-mediated delivery systems: a cancer-catch-cancer strategy in cancer therapy
- PMID: 39811105
- PMCID: PMC11729729
- DOI: 10.1093/rb/rbae135
Homologous-adhering/targeting cell membrane- and cell-mediated delivery systems: a cancer-catch-cancer strategy in cancer therapy
Abstract
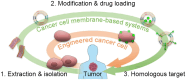
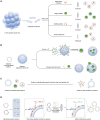
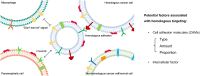
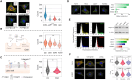
Low tumor enrichment remains a serious and urgent problem for drug delivery in cancer therapy. Accurate targeting of tumor sites is still a critical aim in cancer therapy. Though there have been a variety of delivery strategies to improve the tumor targeting and enrichment, biological barriers still cause most delivered guests to fail or be excreted before they work. Recently, cell membrane-based systems have attracted a huge amount of attention due to their advantages such as easy access, good biocompatibility and immune escape, which contribute to their biomimetic structures and specific surface proteins. Furthermore, cancer cell membrane-based delivery systems are referred to as homologous-targeting function in which they exhibit significantly high adhesion and internalization to homologous-type tumor sites or cells even though the exact mechanism is not entirely revealed. Here, we summarize the sources and characterizations of cancer cell membrane systems, including reconstructed single or hybrid membrane-based nano-/microcarriers, as well as engineered cancer cells. Additionally, advanced applications of these cancer cell membrane systems in cancer therapy are categorized and summarized according to the components of membranes. The potential factors related to homologous targeting of cancer cell membrane-based systems are also discussed. By discussing the applications, challenges and opportunities, we expect the cancer cell membrane-based homologous-targeting systems to have a far-reaching development in preclinic or clinics.
Keywords: cancer therapy; cell membrane; delivery system; homologous targeting; hybrid membrane.
© The Author(s) 2024. Published by Oxford University Press.
Figures


Similar articles
-
Hybrid cell membrane-coated nanoparticles: A multifunctional biomimetic platform for cancer diagnosis and therapy.Acta Biomater. 2020 Aug;112:1-13. doi: 10.1016/j.actbio.2020.05.028. Epub 2020 May 26. Acta Biomater. 2020. PMID: 32470527 Review.
-
Construction of Biomimetic-Responsive Nanocarriers and their Applications in Tumor Targeting.Anticancer Agents Med Chem. 2022;22(12):2255-2273. doi: 10.2174/1871520622666220106105315. Anticancer Agents Med Chem. 2022. PMID: 34994336
-
Less is More: Biomimetic Hybrid Membrane Nanocarriers for Highly Efficient Tumor Targeted Drug Delivery.Small. 2025 Feb;21(6):e2407245. doi: 10.1002/smll.202407245. Epub 2024 Dec 29. Small. 2025. PMID: 39937172
-
Cell Membrane-Coated Biomimetic Nanoparticles in Cancer Treatment.Pharmaceutics. 2024 Apr 12;16(4):531. doi: 10.3390/pharmaceutics16040531. Pharmaceutics. 2024. PMID: 38675192 Free PMC article. Review.
-
Research Progress of Cell Membrane Biomimetic Nanoparticles for Tumor Therapy.Nanoscale Res Lett. 2022 Mar 22;17(1):36. doi: 10.1186/s11671-022-03673-9. Nanoscale Res Lett. 2022. PMID: 35316443 Free PMC article. Review.
Cited by
-
Better together: biomimetic nanomedicines for high performance tumor therapy.Beilstein J Nanotechnol. 2025 Aug 5;16:1246-1276. doi: 10.3762/bjnano.16.92. eCollection 2025. Beilstein J Nanotechnol. 2025. PMID: 40791939 Free PMC article. Review.
-
The Role of ROS in Atherosclerosis and ROS-Based Nanotherapeutics for Atherosclerosis: Atherosclerotic Lesion Targeting, ROS Scavenging, and ROS-Responsive Activity.ACS Omega. 2025 May 23;10(22):22366-22381. doi: 10.1021/acsomega.5c01865. eCollection 2025 Jun 10. ACS Omega. 2025. PMID: 40521521 Free PMC article. Review.
-
Rational design matrix materials for organoid development and application in biomedicine.Regen Biomater. 2025 May 14;12:rbaf038. doi: 10.1093/rb/rbaf038. eCollection 2025. Regen Biomater. 2025. PMID: 40556786 Free PMC article. Review.
References
-
- Ge C, Huang H, Huang F, Yang T, Zhang T, Wu H, Zhou H, Chen Q, Shi Y, Sun Y, Liu L, Wang X, Pearson RB, Cao Y, Kang J, Fu C. Neurokinin-1 receptor is an effective target for treating leukemia by inducing oxidative stress through mitochondrial calcium overload. Proc Natl Acad Sci U S A 2019;116:19635–45. - PMC - PubMed
-
- Li B, Li Q, Qi Z, Li Z, Yan X, Chen Y, Xu X, Pan Q, Chen Y, Huang F, Ping Y. Supramolecular genome editing: targeted delivery and endogenous activation of CRISPR/Cas9 by dynamic Host-Guest recognition. Angew Chem Int Ed Engl 2024;63:e202316323. - PubMed
-
- Li H, Qian X, Mohanram H, Han X, Qi H, Zou G, Yuan F, Miserez A, Liu T, Yang Q, Gao H, Yu J. Self-assembly of peptide nanocapsules by a solvent concentration gradient. Nat Nanotechnol 2024;19:1141–9. - PubMed
-
- Shi Y, Wang X, Meng Y, Ma J, Zhang Q, Shao G, Wang L, Cheng X, Hong X, Wang Y, Yan Z, Cao Y, Kang J, Fu C. A novel mechanism of endoplasmic reticulum stress- and c-Myc-degradation-mediated therapeutic benefits of antineurokinin-1 receptor drugs in colorectal cancer. Adv Sci (Weinh) 2021;8:e2101936. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

