The WOCA negative pressure wound therapy device designed for low resource settings
- PMID: 39811537
- PMCID: PMC11732568
- DOI: 10.1016/j.ohx.2024.e00620
The WOCA negative pressure wound therapy device designed for low resource settings
Abstract
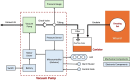
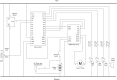
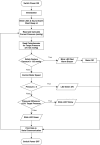
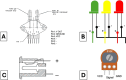
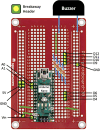
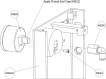
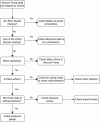
Negative Pressure Wound Therapy (NPWT) is a treatment that promotes healing of chronic wounds. Despite high prevalence of chronic wounds in Low- and Middle-Income Countries (LMICs), NPWT devices are not available nor affordable. This study aims to improve chronic wound care in LMICs by presenting the Wound Care (WOCA) system, designed for building, testing and use in LMICs. Design requirements were formulated using input from literature, ISO standards, and wound care experts. The WOCA design was developed to provide safe, portable, user-friendly and affordable NPWT to patients in LMICs. The design features an adjustable operating pressure ranging from -75 to -125 mmHg, a battery for portability, a 300 ml canister, overflow protection, and system state alarms. An Arduino controls the pressure and monitors the system state. Three prototypes were developed and built in Nepal, and their performance was evaluated. Pressure control was 125 ± 10 % mmHg, internal leakage was 7.5 ± 4.3 mmHg/min, reserve capacity was 189 ± 16.9 ml/min, and overflow protection and alarm systems were effectively working. Prototype cost was approximately 280 USD. The WOCA demonstrates to be a locally producible NPWT device that can safely generate a stable vacuum. Future research will include clinical trials situated in LMICs.
Keywords: Low-cost; Low-resource setting; Medical device; Negative pressure wound therapy; Vacuum-assisted closure therapy; Wound care.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
























References
-
- Mazuz R. Wound care in the developing world - gaps, opportunities, and realities. Curr. Dermatol. Rep. 2019;8(4):199–207. doi: 10.1007/s13671-019-00278-x. - DOI
-
- L.C. Argenta, M.J. Morykwas, Vacuum-assisted closure: a new method for wound control and treatment: clinical experience, Ann. Plast. Surg. 38 (6) (1997) 563-76; discussion 577. https://doi.org/10.1097/00000637-199706000-00002. - PubMed
LinkOut - more resources
Full Text Sources

