The impact of highly effective modulator therapy on sinusitis and dysosmia in young children with cystic fibrosis: a prospective study protocol
- PMID: 39811548
- PMCID: PMC11726580
- DOI: 10.1183/23120541.00137-2024
The impact of highly effective modulator therapy on sinusitis and dysosmia in young children with cystic fibrosis: a prospective study protocol
Abstract
Background: Chronic rhinosinusitis (CRS) and olfactory dysfunction (OD) are prevalent disease complications in people with cystic fibrosis. These understudied comorbidities significantly impact quality of life. The impact of highly effective modulator therapy (HEMT) in young children with cystic fibrosis (YCwCF) on these disease complications is unknown. This proposed study aims to characterise CRS and OD in YCwCF and assess the efficacy of HEMT in improving sinus and olfactory health in this young age group.
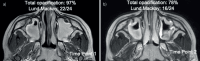
Methods: This six-centre, prospective, observational study will enrol 80 YCwCF aged 2-8 years. Patients are divided into two groups: those receiving HEMT and those not on HEMT based on clinical indication. Both groups undergo sinus magnetic resonance imaging, psychophysical olfactory tests, and complete patient- or parent-reported quality of life surveys over 2 years. Outcomes will be compared before and after initiation of HEMT and between groups. Ethical approval has been obtained for all sites, and this study has been registered on ClinicalTrials.gov (NCT06191640).
Results: Enrolment began in April 2023. 21 participants have been enrolled as of October 2023 with ongoing enrolment at all sites.
Conclusion: This investigation is expected to provide critical insights into the potential benefits of early HEMT initiation in managing CRS and OD in YCwCF. It will assist in developing targeted interventions and contribute to the understanding of HEMT's role in altering the disease course in this demographic.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: C.M. Liu reports an NIDCD grant related to this work. Conflict of interest: J.L. Fischer has nothing to disclose. Conflict of interest: E.T. Zermaick reports that in the last 36 months, she has received grants to her institution from the Cystic Fibrosis Foundation, National Institutes of Health and Vertex Pharmaceuticals Incorporated; has received fees from the Cystic Fibrosis Foundation and Vertex Pharmaceuticals Incorporated related to consultation, participation on advisory boards, and grant review committees; she served as chair of the CFF Therapeutics Development Network steering committee, reviewed grants for CF Canada, and received travel support from the European CF Society Clinical Trials Network for speaking at their annual meeting. Conflict of interest: J.C. Woods reports that in the last 36 months, he has received grants to his institution from the Cystic Fibrosis Foundation, the National Institutes of Health, Vertex Pharmaceuticals Incorporated; and has received fees from Polarean LLC related to consultation on clinical and translational research. Conflict of interest: K.K. Markarian has nothing to disclose. Conflict of interest: S.B. Fain reports that in the last 36 months, he has served as a scientific advisor for Polarean LLC, and received research funding from Polarean LLC, Siemens Healthineers INC, and GE Healthcare INC for development of pulmonary CT and MRI methods. Conflict of interest: D. Froh reports that in the lasts 36 months, she has received grant support from the CF Foundation unrelated to this work, and from Vertex Pharmaceuticals Conflict of interest: S.L. Heltsche reports that in the last 36 months, they have received grant support from the Cystic Fibrosis Foundation; unrelated to this work they have has received grant support from National Institutes of Health and Boehringer Ingelheim, as well as service contracts from Calyx paid to their institution. Conflict of interest: L.R. Hoffman reports that in the past 36 months, they received grant funding from the CF Foundation and the NIH. Conflict of interest: S.M. Humphries declares grants from Boehringer Ingelheim and contracts from Calyx/Perceptive paid to their institution and unrelated to this work. E.L. Kramer reports that in the last 36 months, she has received grant support from the National Institutes of Health and the Cystic Fibrosis Foundation. Conflict of interest: K.L. Ode reports that in the last 36 months, she has received grant support from the National Institutes of Health, the Cystic Fibrosis Foundation, and Rhythm Pharmaceuticals, Inc. unrelated to this work Conflict of interest: M. Lewis participated in an advisory board serving as a consult for Pfizer to discuss RSV vaccines on 20 September 2023. Conflict of interest: D.A. Li reports that in the last 36 months, he has received grant support from the CF Foundation unrelated to this work. Conflict of interest: J. Mata reports that in the last 36 months, he has received grants to his institution from the Cystic Fibrosis Foundation, the National Institutes of Health, the Focused Ultrasound Foundation, iThriv and Polarean LLC; and fees from Polarean LLC related to consultation on clinical and translational research. Conflict of interest: S.S. Milla has nothing to disclose. Conflict of interest: P.J. Niedbalski reports that in the last 36 months, he has received grants from the National Institutes of Health, American Heart Association and Scleroderma Foundation. Conflict of interest: B.D. Sawatzky has nothing to disclose. Conflict of interest: M-S. Sim has nothing to disclose. Conflict of interest: J.S. Sullivan reports that in the last 36 months, she has received honoraria from the American Academy of Pediatrics to serve on the PREP-SA editorial board, unrelated to this work Conflict of interest: A.T. Trout reports that in the last 36 months, he has received grant to his institution from the Cystic Fibrosis Foundation, National Institutes of Health, GE Healthcare, Siemens Healthineers and Perspectum Inc.; and is a consultant for GE Healthcare and serves as an Assistant Editor for Pediatric Radiology. Conflict of interest: C.H. Gross reports that in the last 36 months, he has received grants from the National Institutes of Health, the Cystic Fibrosis Foundation, the Federal Drug Administration; has received fees from Enterprise Therapeutics for providing clinical trial design advice, honoraria from Gilead Sciences to serve as grant review committee chair and from Vertex Pharmaceuticals for speaking at the UK LEAD conference, served as a DSMB Chair for a trial supported by Novartis and the European Commission, and serves as the Deputy Editor of the Annals of the American Thoracic Society; he has stock in Air Therapeutics. Conflict of interest: J.L. Taylor-Cousar reports that faculty in an institution that is part of the CF TDN, she has been a site principal investigator on studies for Vertex, 4DMT, and Eloxx; has consulted/provided clinical trial design advice for Vertex and 4DMT; served as Chair of a Data Monitoring Committee for AbbVie (complete); and has received grant funding from the CFF and NIH; and is a member of the CFF Clinical Research Executive Committee, CFF TDN Sexual Health, Reproduction, and Gender Research Working Group (SHARING), and CFF Racial Justice Working Group, is ATS International Conference Committee Chair-Elect and Respiratory Health Awards Committee Member (Scientific Grant Review and Clinical Problems Programming Committee-complete), and is a member of the Emily's Entourage Scientific Advisory Board, and National Institutes of Health/National Heart, Blood, Lung Institute Clinical Trials Review Study Section. Conflict of interest: D.M. Beswick reports that in the last 36 months, he has received grant support from CF Foundation and American Rhinologic Society; and unrelated to this work, has received grant support from the International Society of Inflammation and Allergy of the Nose and the American Rhinologic Society; honoraria and consulting fees from Amgen and, on medicolegal cases, at Garner Health (equity).
Figures


References
-
- Cystic Fibrosis Foundation. 2022. Patient Registry Annual Data Report. Date last accessed: 15 January 2024. www.cff.org/medical-professionals/patient-registry.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
