Cost-effectiveness of follow-up algorithms for chronic thromboembolic pulmonary hypertension in pulmonary embolism survivors
- PMID: 39811556
- PMCID: PMC11726578
- DOI: 10.1183/23120541.00575-2024
Cost-effectiveness of follow-up algorithms for chronic thromboembolic pulmonary hypertension in pulmonary embolism survivors
Abstract
Introduction: Achieving an early diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH) in pulmonary embolism (PE) survivors results in better quality of life and survival. Importantly, dedicated follow-up strategies to achieve an earlier CTEPH diagnosis involve costs that were not explicitly incorporated in the models assessing their cost-effectiveness. We performed an economic evaluation of 11 distinct PE follow-up algorithms to determine which should be preferred.
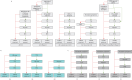
Materials and methods: 11 different PE follow-up algorithms and one hypothetical scenario without a dedicated CTEPH follow-up algorithm were included in a Markov model. Diagnostic accuracy of consecutive tests was estimated from patient-level data of the InShape II study (n=424). The lifelong costs per CTEPH patient were compared and related to quality-adjusted life-years (QALYs) for each scenario.
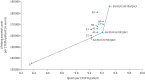
Results: Compared to not performing dedicated follow-up, the integrated follow-up algorithms are associated with an estimated increase of 0.89-1.2 QALYs against an incremental cost-effectiveness ratio (ICER) of EUR 25 700-46 300 per QALY per CTEPH patient. When comparing different algorithms with each other, the maximum differences were 0.27 QALYs and EUR 27 600. The most cost-effective algorithm was the InShape IV algorithm, with an ICER of EUR 26 700 per QALY compared to the next best algorithm.
Conclusion: Subjecting all PE survivors to any of the currently established dedicated follow-up algorithms to detect CTEPH is cost-effective and preferred above not performing a dedicated follow-up, evaluated against the Dutch acceptability threshold of EUR 50 000 per QALY. The model can be used to identify the locally preferred algorithm from an economical point-of-view within local logistical possibilities.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: S. Barco received research support from Boston Scientific, Medtronic, Concept Medical, Sanofi and Novartis, all outside this manuscript. Conflict of interest: M. Delcroix received consulting fees from Actelion/Janssen/J&J, Acceleron/MSD, Gossamer and Ferrer, all outside the submitted work. Conflict of interest: L. Jara-Palomares reports grants from Daichii, Rovi, GlaxoSmithKline, BMS, Johnson and Johnson, Leo Pharma and MSD, all outside the submitted work. Conflict of interest: S.V. Konstantinides reports grants or contacts from Daiichi-Sankyo, and consulting fees from Boston Scientific, Inari Medical, Bayer AG, Penumbra Inc., Daiichi Sankyo, all outside this manuscript. Conflict of interest: F.A. Klok received research support from Bayer, BMS, BSCI, AstraZeneca, MSD, Leo Pharma, Actelion, Farm-X, The Netherlands Organisation for Health Research and Development, The Dutch Thrombosis Foundation, The Dutch Heart Foundation and the Horizon Europe Program, all outside this manuscript. Conflict of interest: All the other authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
