The Possible Ameliorative Effect of Echinacea, Ginger, and Their Combination on Experimentally Induced Diabetic Nephropathy in a Rat Model: Histological and Immunohistochemical Study
- PMID: 39811596
- PMCID: PMC11729022
- DOI: 10.4103/jmau.jmau_62_22
The Possible Ameliorative Effect of Echinacea, Ginger, and Their Combination on Experimentally Induced Diabetic Nephropathy in a Rat Model: Histological and Immunohistochemical Study
Abstract
Background: Diabetes represents a chronic disease characterized by hyperglycemia. Several changes in the renal functions had been detected in diabetic patients.
Aim of the work: This study was conducted to compare the possible ameliorative role of both ginger and Echinacea either alone or in combination upon experimentally induced diabetic nephropathy.
Materials and methods: Sixty adult male albino rats were used in this study. Rats were divided into three groups. Control (group I) included 20 rats. Diabetic group (group II) included 10 rats. Group III included 30 rats subdivided into three subgroups 10 animals each: Subgroup IIIa diabetic treated with 100 mg/kg Echinacea for 30 days. Subgroup IIIb diabetic treated with 400 mg/kg ginger for 30 days orally. Subgroup IIIc diabetic treated with both 100 mg/kg Echinacea and 400 mg/kg ginger for 30 days orally. Hemotoxylin and eosin staining, Periodic acid Schiff and Masson trichrome were done. Ultrastructural examination was done. Immunohistochemical markers used were caspase-3 for apoptosis and CD68 for macrophages. Morphometric and statistical analyses were done.
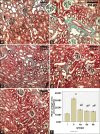
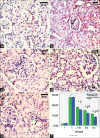
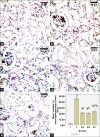
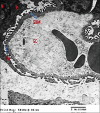
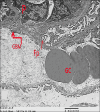
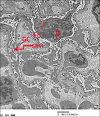
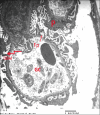
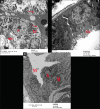
Results: Diabetes caused a significant increase in collagen fibers in the renal cortex, the caspase-3 expression as well as the number of macrophages. Ultrastructurally, there was an irregularly thickened glomerular basement membrane and effacement of podocytes. Ginger treatment alone or in combination with Echinacea exhibited more pronounced improvement of diabetes-induced degenerative changes and a significant decrease in collagen fibers, the caspase-3 expression as well as the number of macrophages compared to Echinacea alone.
Conclusion: Ginger treatment alone or in combination with Echinacea exhibited more pronounced improvement in diabetes nephropathy.
Keywords: Caspase-3; DC68; Diabetic nephropathy; Echinacea; ginger.
Copyright: © 2023 Journal of Microscopy and Ultrastructure.
Conflict of interest statement
There are no conflicts of interest.
Figures








References
-
- Valencia WM, Florez H. How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ. 2017;356:i6505. - PubMed
-
- Samsu N. Diabetic nephropathy: Challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:17. https://doi.org/10.1155/2021/1497449. - PMC - PubMed
-
- Faridi Esfanjani A, Jafari SM. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf B Biointerfaces. 2016;146:532–43. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
