An antisense-long-noncoding-RNA modulates p75NTR expression levels during neuronal polarization
- PMID: 39811648
- PMCID: PMC11730960
- DOI: 10.1016/j.isci.2024.111566
An antisense-long-noncoding-RNA modulates p75NTR expression levels during neuronal polarization
Abstract
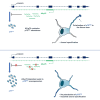
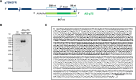
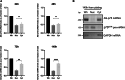
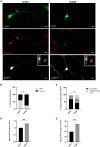
Proper polarization of newly generated neurons is a critical process for neural network formation and brain development. The pan-neurotrophin p75NTR receptor plays a key role in this process localizing asymmetrically in one of the differentiating neurites and specifying its axonal identity in response to neurotrophins. During axonal specification, p75NTR levels are transiently modulated, yet the molecular mechanisms underlying this process are not known. Here, we identified a previously uncharacterized natural antisense transcript, AS-p75, encoded within the p75NGFR mouse gene. Using an in vitro model of polarizing murine neurons, we found that AS-p75 and p75NTR display divergent expression profiles and that p75NTR expression levels increase upon competition or depletion of AS-p75, indicating that AS-p75 is a negative regulator of p75NTR expression. Depletion of AS-p75 also results in altered p75NTR subcellular distribution and affects the polarization process. Overall, our data uncovered AS-p75 as a modulator of p75NTR expression, offering new insights into the regulation of this neurotrophin receptor during in vitro neuronal polarization.
Keywords: Cellular neuroscience; Molecular neuroscience.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

