CD137 agonism enhances anti-PD1 induced activation of expanded CD8+ T cell clones in a neoadjuvant pancreatic cancer clinical trial
- PMID: 39811671
- PMCID: PMC11730579
- DOI: 10.1016/j.isci.2024.111569
CD137 agonism enhances anti-PD1 induced activation of expanded CD8+ T cell clones in a neoadjuvant pancreatic cancer clinical trial
Abstract
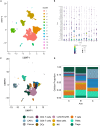
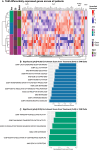
Successful pancreatic ductal adenocarcinoma (PDAC) immunotherapy requires therapeutic combinations that induce quality T cells. Tumor microenvironment (TME) analysis following therapeutic interventions can identify response mechanisms, informing design of effective combinations. We provide a reference single-cell dataset from tumor-infiltrating leukocytes (TILs) from a human neoadjuvant clinical trial comparing the granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting allogeneic PDAC vaccine GVAX alone, in combination with anti-PD1 or with both anti-PD1 and CD137 agonist. Treatment with GVAX and anti-PD-1 led to increased CD8+ T cell activation and expression of cytoskeletal and extracellular matrix (ECM)-interacting components. Addition of CD137 agonist increased abundance of clonally expanded CD8+ T cells and increased immunosuppressive TREM2 signaling in tumor associated macrophages (TAMs), identified by comparison of ligand-receptor networks, corresponding to changes in metabolism and ECM interactions. These findings associate therapy with GVAX, anti-PD1, and CD137 agonist with enhanced CD8+ T cell function while inducing alternative immunosuppressive pathways in patients with PDAC.
Keywords: Health sciences; Internal medicine; Medical specialty; Medicine; Oncology.
Conflict of interest statement
L.Z. receives grant support from Bristol Myers Squibb, Merck, Astrazeneca, iTeos, Amgen, NovaRock, Inxmed, and Halozyme. L.Z. is a paid consultant/advisory Board Member at Biosion, Alphamab, NovaRock, Ambrx, Akrevia/Xilio, QED, Natera, Novagenesis, Snow Lake Capitals, BioArdis, Amberstone Biosciences, Tempus, Pfizer, Tavotek Lab, Clinical Trial Options, LLC, and Mingruizhiyao. L.Z. holds shares at Alphamab, Amberstone, and Mingruizhiyao. E.M.J. reports other support from Abmeta and Adventris, personal fees from Achilles, Dragonfly, Mestag, The Medical Home Group, and Surgtx, other support from Parker Institute, grants and other support from the Lustgarten Foundation, Genentech, BMS, and Break Through Cancer outside the submitted work. E.S.C. receives grant support from Affimed GmbH, NextCure, Pfizer, Haystack Oncology, Regeneron and is a consultant for Seres Therapeutics and SIRTex. R.A.A. receives grant support from Bristol-Meyer Squibb, RAPT Therapeutics. R.A.A. is a paid consultant for Bristol-Meyer Squibb, Merck, and Astrazeneca. S.Y. reports grants from NIH and Maryland Cigarette Restitution Fund during the conduct of the study, grants and personal fees from Cepheid, other support from Digital Harmonic, and grants from Janssen and Bristol Myers Squibb outside the submitted work. J.H.E. was previously a consultant and holds equity in Unity Biotechnology, Aegeria Soft Tissue and is an advisor for Tessera Therapeutics, HapInScience, Regenity, and Font Bio. W.J.H. has patent royalties from Rodeo/Amgen, grants from Sanofi and NeoTX, and speaking/travel honoraria from Exelixis and Standard BioTools. J.W.Z. reports grant funding from Genentech.
Figures




References
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA Cancer J. Clin. 2022;72:7–33. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

