Increased functional potency of multi-edited CAR-T cells manufactured by a non-viral transfection system
- PMID: 39811687
- PMCID: PMC11730244
- DOI: 10.1016/j.omtm.2024.101389
Increased functional potency of multi-edited CAR-T cells manufactured by a non-viral transfection system
Abstract
Chimeric antigen receptor (CAR)-T cell therapy represents a breakthrough for the treatment of hematological malignancies. However, to treat solid tumors and certain hematologic cancers, next-generation CAR-T cells require further genetic modifications to overcome some of the current limitations. Improving manufacturing processes to preserve cell health and function of edited T cells is equally critical. Here, we report that Solupore, a Good Manufacturing Practice-aligned, non-viral physicochemical transfection system, can be used to manufacture multi-edited CAR-T cells using CRISPR-Cas9 ribonucleoproteins while maintaining robust cell functionality. When compared to electroporation, an industry standard, T cells that were triple edited using Solupore had reduced levels of apoptosis and maintained similar proportions of stem cell memory T cells with higher oxidative phosphorylation levels. Following lentiviral transduction with a CD19 CAR, and subsequent cryopreservation, triple-edited CAR-T cells manufactured using Solupore demonstrated improved immunological synapse strength to target CD19+ Raji cells and enhanced cellular cytotoxicity compared with electroporated CAR-T cells. In an in vivo mouse model (NSG), Solupore triple-edited CAR-T cells enhanced tumor growth inhibition by more than 30-fold compared to electroporated cells.
Keywords: CAR-T; CAR-T manufacturing; Solupore; adoptive cell therapy; cell avidity; cell cytotoxicity; genetic engineering; metabolism; non-viral transfection; stem cell memory.
© 2024 The Author(s).
Conflict of interest statement
All authors are current or former Avectas employees. Avectas has filed patents covering the Solupore technology described in this paper. M.W.L. is a member of the scientific advisory board of Avectas and supervised experiments performed in the Royal Free Hospital, London and UCL. Avectas has received funding for elements of this work under the Disruptive Technologies Innovation Fund (DTIF), owned by the Department of Enterprise, Trade, and Employment under Call 3, DTIF reference number DT2020224.
Figures
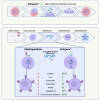


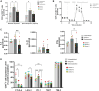
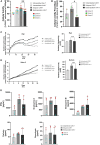


References
-
- Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N., et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. - PMC - PubMed
LinkOut - more resources
Full Text Sources

