Cathepsin-D and outcomes in peripartum cardiomyopathy: Results from IPAC
- PMID: 39811721
- PMCID: PMC11731516
- DOI: 10.1016/j.ahjo.2024.100489
Cathepsin-D and outcomes in peripartum cardiomyopathy: Results from IPAC
Abstract
Objective: Evaluate the relationship of cathepsin-D (CD) on disease severity and clinical outcomes for women with peripartum cardiomyopathy.
Background: Cathepsin-D is a protease released during oxidative stress that cleaves prolactin (PRL) generating a 16 kDa fragment that is pro-apoptotic, anti-angiogenic, and has been implicated in the pathogenesis of peripartum cardiomyopathy (PPCM).
Methods: In 99 women with newly diagnosed PPCM enrolled in the Investigation in Pregnancy Associated Cardiomyopathy (IPAC) study, CD levels were assessed by ELISA from serum obtained at study entry. Left ventricular ejection fraction (LVEF) was assessed by echocardiography at entry, 6, and 12-months. CD levels were compared to healthy PP and non-PP controls. Survival free from major cardiovascular events (death, transplantation, or left ventricular assist device) was determined up to 12 months post-partum (PP).
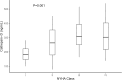
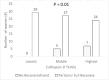
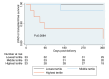
Results: Mean age was 30 ± 6 years, with a baseline LVEF of 34 % ± 10. Cathepsin-D levels were higher in PPCM women (278 ± 114 ng/ml) than in healthy PP (190 ± 74, p = 0.02) and healthy non-PP controls (136 ± 79, p < 0.001). There was no association of CD with age, breastfeeding status, or time from delivery to the presentation. Cathepsin-D levels were higher in women with higher New York Heart Association (NYHA) functional class (p = 0.001). Higher tertiles of CD levels were associated with lower event-free survival (p = 0.008).
Conclusions: In this prospective cohort of women with PPCM, higher CD levels at the time of diagnosis were associated with worse symptoms, less recovery of LVEF, and worse clinical outcomes. Circulating CD may contribute to the development of PPCM and influence disease severity, myocardial recovery, and clinical outcomes.
Keywords: Cardiomyopathies; Heart failure; Peripartum cardiomyopathy; Pregnancy.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Arany Z., Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397–1409. - PubMed
-
- Gunderson E.P., Croen L.A., Chiang V., Yoshida C.K., Walton D., Go A.S. Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet. Gynecol. 2011;118:583–591. - PubMed
-
- Ansari A.A., Fett J.D., Carraway R.E., Mayne A.E., Onlamoon N., Sundstrom J.B. Autoimmune mechanisms as the basis for human peripartum cardiomyopathy. Clin Rev Allergy Immunol. 2002;23:301–324. - PubMed
-
- Bültmann B.D., Klingel K., Näbauer M., Wallwiener D., Kandolf R. High prevalence of viral genomes and inflammation in peripartum cardiomyopathy. Am. J. Obstet. Gynecol. 2005;193:363–365. - PubMed
-
- Lamparter S., Pankuweit S., Maisch B. Clinical and immunologic characteristics in peripartum cardiomyopathy. Int. J. Cardiol. 2007;118:14–20. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

