Unilateral Versus Bilateral Endoscopic Resection of Olfactory Neuroblastoma: Pooled Analysis From Prospective and Retrospective Multicenter Data
- PMID: 39811891
- PMCID: PMC12135456
- DOI: 10.1002/alr.23531
Unilateral Versus Bilateral Endoscopic Resection of Olfactory Neuroblastoma: Pooled Analysis From Prospective and Retrospective Multicenter Data
Abstract
Background: Olfactory neuroblastoma (ONB) is a rare sinonasal malignancy primarily treated with surgery. For tumors arising from the olfactory area, traditional treatment involves transcribriform resection of the anterior cranial fossa. Surgery can be performed with unilateral or bilateral resection depending on extent of involvement; however, there are currently no studies comparing outcomes between the two.
Methods: Prospective and retrospective data on primary ONB patients were collected from a multicenter registry involving eight academic sites. Propensity score matching (PSM) was used to create patient cohorts with similar baseline characteristics. Cox proportional hazards and Kaplan Meier analyses assessed overall survival (OS). Logistic regression assessed associations between extent of resection (unilateral versus bilateral) and tumor recurrence or postoperative cerebrospinal fluid (CSF) leak.
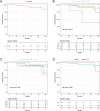
Results: A total of 187 ONB patients (47.6% female) with an average age of 53.6 ± 15.1 years were analyzed. Most tumors were unilateral (81.3%) and predominantly Kadish C (58.3%) or Hyams II (55.8%). Overall, 56.7% of patients underwent bilateral resection. Fifty-four patients experienced tumor recurrence and nine had postoperative CSF leaks. Following PSM (n = 45/group), extent of resection was not associated with mortality (hazard ratio [HR] 1.73; p = 0.407) or OS (p = 0.400). There was no association between extent of resection and recurrence (odds ratio [OR] 0.90; p = 0.814) or postoperative CSF leak (OR 1.54; p = 0.647).
Conclusions: For ONB tumors where unilateral resection may be feasible and oncologically sound, the decision for unilateral versus bilateral resection showed no significant effect on survival, recurrence, or postoperative CSF leak. Oncologic outcomes may be comparable when resection is tailored to individual patient and tumor characteristics.
Keywords: endoscopic surgery; esthesioneuroblastoma; olfactory neuroblastoma; outcomes; resection; survival.
© 2025 The Author(s). International Forum of Allergy & Rhinology published by Wiley Periodicals LLC on behalf of American Academy of Otolaryngic Allergy and American Rhinologic Society.
Conflict of interest statement
N.D.A. served as consultant for Acclarent, Optinose, and 3‐D Matrix. In the last 36 months, D.M.B. has received grant support from NIH/NHLBI, CF Foundation, International Society of Inflammation and Allergy of the Nose, and the American Rhinologic Society CORE/Sue Ann and John L. Weinberg Foundation; honoraria from sources including from National Jewish Health; and consulting fees from Amgen on medicolegal cases and from Garner Health (equity). G.W.C. served as consultant for Tissium, LLC, and Medtronic ENT. E.H.C. served as consultant for Sanofi/Regeneron and received grant funding from NIH. P.H.H. served as consultant for Stryker and Medtronic, and consultant/equity for Slate Therapeutics. He also holds equity in SoundHealth. J.V.N. served as consultant for Aerin Medical and also holds equity/patent with SpirAir LLC. He has also received grant support from the National Institutes of Health (NIH). Z.M.P. served as consultant/advisor for Medtronic, Optinose, Regeneron/Sanofi, Ethicon J&J, Mediflix, Consumer Medical, and SoundHealth. She also holds equity in Olfera Therapeutics, CORE/Sue Ann, and John L. Weinberg Foundation. She received honoraria and consulting fees from Amgen, on medicolegal cases, and Garner Health (equity). C.H.S. served as consultant for SPIWay and LLC, and holds equity in Respair, Inc. E.C.K. served as consultant for Stryker and 3‐D Matrix, and received royalties from Springer. E.W.W. received grant support from NIH/NIAID. All other authors have no conflicts of interest.
Figures

References
-
- Limaiem F. and Das J. M., “Esthesioneuroblastoma,” in StatPearls (Treasure Island (FL): StatPearls Publishing, 2024), http://www.ncbi.nlm.nih.gov/books/NBK539694/.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

