Leveraging Optical Anisotropy of the Morpho Butterfly Wing for Quantitative, Stain-Free, and Contact-Free Assessment of Biological Tissue Microstructures
- PMID: 39811986
- PMCID: PMC11937990
- DOI: 10.1002/adma.202407728
Leveraging Optical Anisotropy of the Morpho Butterfly Wing for Quantitative, Stain-Free, and Contact-Free Assessment of Biological Tissue Microstructures
Abstract
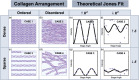
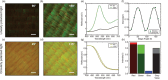
Changes in the density and organization of fibrous biological tissues often accompany the progression of serious diseases ranging from fibrosis to neurodegenerative diseases, heart disease and cancer. However, challenges in cost, complexity, or precision faced by existing imaging methodologies and materials pose barriers to elucidating the role of tissue microstructure in disease. Here, we leverage the intrinsic optical anisotropy of the Morpho butterfly wing and introduce Morpho-Enhanced Polarized Light Microscopy (MorE-PoL), a stain- and contact-free imaging platform that enhances and quantifies the birefringent material properties of fibrous biological tissues. We develop a mathematical model, based on Jones calculus, which describes fibrous tissue density and organization. As a representative example, we analyzed collagen-dense and collagen-sparse human breast cancer tissue sections and leverage our technique to assess the microstructural properties of distinct regions of interest. We compare our results with conventional Hematoxylin and Eosin (H&E) staining procedures and second harmonic generation (SHG) microscopy for fibrillar collagen detection. Our findings demonstrate that our MorE-PoL technique provides a robust, quantitative, and accessible route toward analyzing biological tissue microstructures, with great potential for application to a broad range of biological materials.
Keywords: histopathology; morpho butterfly wing; photonic surface; polarized light; structural color; tissue imaging; tissue microstructure.
© 2025 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Update of
-
Leveraging Optical Anisotropy of the Morpho Butterfly Wing for Quantitative, Stain-Free, and Contact-Free Assessment of Biological Tissue Microstructures.ArXiv [Preprint]. 2024 May 30:arXiv:2405.19632v1. ArXiv. 2024. Update in: Adv Mater. 2025 Mar;37(12):e2407728. doi: 10.1002/adma.202407728. PMID: 38855553 Free PMC article. Updated. Preprint.
References
-
- Haddadin Z., Pike T., Moses J. J., Poulikakos L. V., J. Mater. Chem. C 2021, 9, 11619.
-
- Lu S.‐Y., Chipman R. A., JOSA A 1996, 13, 1106.
-
- Ghosh N., Wood M. F. G., Li S., Weisel R. D., Wilson B. C., Li R.‐K., Vitkin I. A., J. Biophotonics 2009, 2, 145. - PubMed
-
- Cowin S. C., J. Biomech. Eng. 2000, 122, 553. - PubMed
MeSH terms
Substances
Grants and funding
- R01 CA236386/CA/NCI NIH HHS/United States
- R01 CA262794/CA/NCI NIH HHS/United States
- OpticaAmplifyScholarship/Optica Foundation
- R01 CA268179/CA/NCI NIH HHS/United States
- T32FT4922/Tobacco-Related Disease Research Program
- R01CA268179/Division of Cancer Prevention, National Cancer Institute
- Optica Women's Scholarship/Optica Foundation
- R01CA236386/Division of Cancer Prevention, National Cancer Institute
- R01 CA174869/CA/NCI NIH HHS/United States
- DGE-2038238/National Science Foundation Graduate Research Fellowship Program
- RO1CA262794/Division of Cancer Prevention, National Cancer Institute
- Selected Professions Fellowship/American Association of University Women
- 30155266/Arnold and Mabel Beckman Foundation
- R01CA174869/Division of Cancer Prevention, National Cancer Institute
LinkOut - more resources
Full Text Sources
Research Materials

