Pharmacovigilance Pregnancy Data in a Population of Japanese Patients With Chronic Inflammatory Disease Exposed to Certolizumab Pegol
- PMID: 39812078
- PMCID: PMC11733829
- DOI: 10.1111/1756-185X.70048
Pharmacovigilance Pregnancy Data in a Population of Japanese Patients With Chronic Inflammatory Disease Exposed to Certolizumab Pegol
Abstract
Aim: Uncontrolled chronic inflammatory diseases (CIDs) before, during, and after pregnancy, as well as some CID medications, can increase the risk of impaired fertility in addition to adverse maternal/pregnancy outcomes in women of childbearing age. We report pregnancy outcomes from prospectively reported pregnancies in Japanese women treated with certolizumab pegol (CZP).
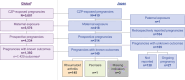
Methods: Data from July 2001 to November 2020 on CZP-exposed pregnancies from the CZP Pharmacovigilance safety database were reviewed. Pregnancy outcomes analyzed included live birth, ectopic pregnancy, abortion (miscarriage or medically indicated/elective), and stillbirth. Congenital anomalies (major and minor), preterm delivery, and low birth weight were also examined.
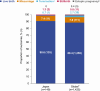
Results: Among 149 prospective pregnancies with maternal CZP exposure and known outcomes identified in Japanese women, 111/149 (74.5%) involved at least first-trimester exposure and 53/149 (35.6%) were exposed in all trimesters; 135/149 (90.6%) live births, 12/149 (8.1%) abortions (11 miscarriages, one elective termination), 2/149 (1.3%) stillbirths, no ectopic pregnancies reported. One (0.7%) infant, whose mother had first-trimester exposure, manifested a minor congenital anomaly (accessory auricle). There were no major congenital anomalies. Among live births, 3/135 (2.2%) were preterm and 10/135 (7.4%) had low birth weight.
Conclusion: The safety profile of CZP in pregnant Japanese women was consistent with published global data.
Keywords: Certolizumab pegol; Japanese patients; TNFi; congenital anomalies; pregnancy outcomes.
© 2025 The Author(s). International Journal of Rheumatic Diseases published by Asia Pacific League of Associations for Rheumatology and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Figures

References
-
- Kavanaugh A., Cush J. J., Ahmed M. S., et al., “Proceedings From the American College of Rheumatology Reproductive Health Summit: The Management of Fertility, Pregnancy, and Lactation in Women With Autoimmune and Systemic Inflammatory Diseases,” Arthritis Care & Research 67, no. 3 (2015): 313–325, 10.1002/acr.22516. - DOI - PubMed
-
- Meissner Y., Rudi T., Fischer‐Betz R., and Strangfeld A., “Pregnancy in Women With Psoriatic Arthritis: A Systematic Literature Review of Disease Activity and Adverse Pregnancy Outcomes,” Seminars in Arthritis and Rheumatism 51, no. 3 (2021): 530–538, 10.1016/j.semarthrit.2021.04.003. - DOI - PubMed
-
- Maguire S., Wilson F., Gallagher P., Mohamed M. M. S., Maher S., and O'Shea F., “What to Expect When Women With Axial Spondyloarthritis Are Expecting: Prevalence of Complications of Pregnancies in Women With Axial Spondyloarthritis,” Seminars in Arthritis and Rheumatism 54 (2022): 151993, 10.1016/j.semarthrit.2022.151993. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

