Complementary cognitive roles for D2-MSNs and D1-MSNs during interval timing
- PMID: 39812105
- PMCID: PMC11735027
- DOI: 10.7554/eLife.96287
Complementary cognitive roles for D2-MSNs and D1-MSNs during interval timing
Abstract
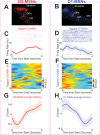
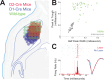
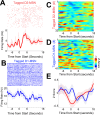
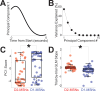
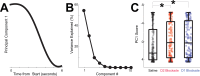
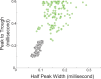
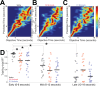
The role of striatal pathways in cognitive processing is unclear. We studied dorsomedial striatal cognitive processing during interval timing, an elementary cognitive task that requires mice to estimate intervals of several seconds and involves working memory for temporal rules as well as attention to the passage of time. We harnessed optogenetic tagging to record from striatal D2-dopamine receptor-expressing medium spiny neurons (D2-MSNs) in the indirect pathway and from D1-dopamine receptor-expressing MSNs (D1-MSNs) in the direct pathway. We found that D2-MSNs and D1-MSNs exhibited distinct dynamics over temporal intervals as quantified by principal component analyses and trial-by-trial generalized linear models. MSN recordings helped construct and constrain a four-parameter drift-diffusion computational model in which MSN ensemble activity represented the accumulation of temporal evidence. This model predicted that disrupting either D2-MSNs or D1-MSNs would increase interval timing response times and alter MSN firing. In line with this prediction, we found that optogenetic inhibition or pharmacological disruption of either D2-MSNs or D1-MSNs increased interval timing response times. Pharmacologically disrupting D2-MSNs or D1-MSNs also changed MSN dynamics and degraded trial-by-trial temporal decoding. Together, our findings demonstrate that D2-MSNs and D1-MSNs had opposing dynamics yet played complementary cognitive roles, implying that striatal direct and indirect pathways work together to shape temporal control of action. These data provide novel insight into basal ganglia cognitive operations beyond movement and have implications for human striatal diseases and therapies targeting striatal pathways.
Keywords: dopamine; mouse; neuroscience; striatum; timing.
© 2024, Bruce et al.
Conflict of interest statement
RB, MW, AB, RV, CJ, KS, HS, YK, RC, KN No competing interests declared
Figures


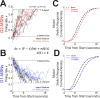
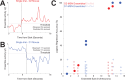
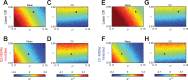
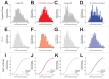

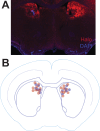
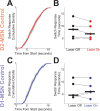

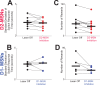
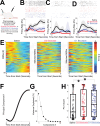

Update of
-
Complementary cognitive roles for D2-MSNs and D1-MSNs during interval timing.bioRxiv [Preprint]. 2024 Sep 6:2023.07.25.550569. doi: 10.1101/2023.07.25.550569. bioRxiv. 2024. Update in: Elife. 2025 Jan 15;13:RP96287. doi: 10.7554/eLife.96287. PMID: 37546735 Free PMC article. Updated. Preprint.
Similar articles
-
Complementary cognitive roles for D2-MSNs and D1-MSNs during interval timing.bioRxiv [Preprint]. 2024 Sep 6:2023.07.25.550569. doi: 10.1101/2023.07.25.550569. bioRxiv. 2024. Update in: Elife. 2025 Jan 15;13:RP96287. doi: 10.7554/eLife.96287. PMID: 37546735 Free PMC article. Updated. Preprint.
-
Sustained fentanyl exposure inhibits neuronal activity in dissociated striatal neuronal-glial cocultures through actions independent of opioid receptors.J Neurophysiol. 2024 Sep 1;132(3):1056-1073. doi: 10.1152/jn.00444.2023. Epub 2024 Aug 7. J Neurophysiol. 2024. PMID: 39110896
-
Divergent Roles of Nucleus Accumbens D1- and D2-MSNs in Regulating Hedonic Feeding.bioRxiv [Preprint]. 2025 Jul 29:2025.07.23.666390. doi: 10.1101/2025.07.23.666390. bioRxiv. 2025. PMID: 40766710 Free PMC article. Preprint.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Long-term, cell type-specific effects of prenatal stress on dorsal striatum and relevant behaviors in mice.bioRxiv [Preprint]. 2024 Dec 27:2024.12.27.627207. doi: 10.1101/2024.12.27.627207. bioRxiv. 2024. PMID: 39763907 Free PMC article. Preprint.
-
Amphetamine increases timing variability by degrading prefrontal temporal encoding.Neuropharmacology. 2025 Sep 1;275:110486. doi: 10.1016/j.neuropharm.2025.110486. Epub 2025 May 3. Neuropharmacology. 2025. PMID: 40324651
References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

