Critical Issues in the Management of CRMS/CFSPID Children: A National Real-World Survey
- PMID: 39812351
- PMCID: PMC11734379
- DOI: 10.1002/ppul.27483
Critical Issues in the Management of CRMS/CFSPID Children: A National Real-World Survey
Abstract
Background: Notwithstanding guidance from the European Cystic Fibrosis (CF) Society (ECFS) neonatal screening (NBS) working group, significant variation persists in the evaluation and management of Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID) subjects, leaving many aspects of care under debate. This study reports the results of a national survey investigating management and treatment approaches of pre-school CFSPIDs in Italy.
Methods: In February 2024, a comprehensive questionnaire was distributed to all Italian CF centers. The survey explored various aspects of CFSPID management in the year 2023, including patient visit schedules, sweat tests (ST) timing, screening procedures, therapeutic interventions, and discharge criteria. Data on regional NBS protocols, number of CFSPID cases, and CF:CFSPID ratio were also collected.
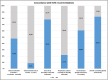
Results: By December 31, 2023, CF Italian centers were following 522 CFSPIDs. In 2023, CF NBS identified 85 CF and 68 CFSPID cases, resulting in a CF:CFSPID ratio of 1.25:1. Seven centers diagnosed more CFSPID than CF, with the lowest CF:CFSPID ratio being 0.20:1. A quarter of all centers reported management plans that deviated widely from ECFS guidelines. Respiratory cultures were performed in 16 (69.6%) centers in the absence of symptoms. Nine (38.9%) prescribed antibiotics in any case of positive Pseudomonas aeruginosa cultures, including first detections and asymptomatic subjects. Spirometries were performed by 14/23 centers (60.9%) in procedure-competent children at each visit. Follow up care continued after age 6 for all CFSPIDs in 15 (65.2%) centers regardless of age, genotype or ST results. A diagnosis of CF was established based on repeated pathological STs and/or multiorgan involvement. Children with STs in intermediate range and mono-organ involvement were classified as CFTR-related disorders (CFTR-RD).
Conclusions: Despite available data on clinical course and recommendations on management of CFSPIDs, different approaches persist in clinical practice. Further efforts should be considered to disseminate and encourage adherence to international guidelines.
Keywords: CFSPID; cystic fibrosis; neonatal screening; outcomes; sweat test.
© 2025 The Author(s). Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Kerem E., Orenti A., Adamoli A., Hatziagorou E., Naehrlich L., and Sermet‐Gaudelus I., “Cystic Fibrosis in Europe: Improved Lung Function and Longevity – Reasons for Cautious Optimism, but Challenges Remain,” European Respiratory Journal 63, no. 3 (2024): 2301241, 10.1183/13993003.01241-2023. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

