Real-World Effectiveness of Long-Acting Injectable and Oral Antipsychotic Agents in US Medicare Patients with Schizophrenia
- PMID: 39812753
- PMCID: PMC11787181
- DOI: 10.1007/s12325-024-03075-6
Real-World Effectiveness of Long-Acting Injectable and Oral Antipsychotic Agents in US Medicare Patients with Schizophrenia
Abstract
Introduction: Daily oral antipsychotics (OAPs) are the mainstay of schizophrenia treatment; however, long-acting injectable antipsychotics (LAIs) are associated with better treatment adherence and improved outcomes.
Methods: This study assessed the real-world comparative effectiveness of LAIs and daily OAPs using claims data from a nationally representative sample of fee-for-service Medicare beneficiaries with schizophrenia. Antipsychotic discontinuation, psychiatric hospitalization, and treatment failure were compared relative to different reference groups using within-individual Cox regression models.
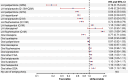
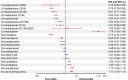
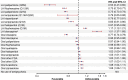
Results: The study included 152,835 patients (mean age, 53.5 years; 54.0% male and 61.5% white). LAIs when grouped by dosing intervals were associated with significantly lower risk of antipsychotic discontinuation (hazard ratios [HRs] 0.27-0.69), psychiatric hospitalization (HRs 0.76-0.88), and treatment failure (HRs 0.55-0.74) compared with OAPs. When LAIs of different dosing intervals and OAPs were broken out by type of agent and compared with oral risperidone, second-generation LAIs, specifically LAI paliperidone (every 3 months [Q3M] and monthly [Q1M]), LAI aripiprazole (Q1M), and LAI risperidone (primarily every 2 weeks), had a significantly lower risk of antipsychotic discontinuation (HRs 0.19-0.67), psychiatric hospitalization (HRs 0.76-0.91), and treatment failure (HRs 0.53-0.85). Second-generation LAI paliperidone (Q3M) had the lowest risk for negative outcomes relative to OAPs; this effect was maintained when the reference group was changed to oral risperidone, LAI risperidone, LAI aripiprazole (Q1M), and LAI haloperidol (Q1M) (33-47% lower risk).
Conclusion: Efforts are needed to enhance identification of appropriate candidates for LAIs and increase their uptake, especially longer dosing interval LAIs, in the Medicare population.
Keywords: Comparative effectiveness; Efficacy; First- and second-generation antipsychotics; Hospitalization; Persistence; Real-world outcomes; Relapse; Schizophrenia; Treatment discontinuation; Treatment failure.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Pengxiang Li reported receiving personal fees from Cobbs Creek Healthcare and SKB Consulting Inc, all unrelated to the submitted work. Zhi Geng has nothing to disclose. Carmela Benson and Charmi Patel reported being employees of Janssen Scientific Affairs, LLC and stockholders of Johnson and Johnson. Jalpa Doshi reported receiving grants from Janssen Scientific Affairs, LLC during the conduct of the study; personal fees from AbbVie, Acadia, Janssen, Merck, Otsuka, and Takeda; and grants from Merck and Spark Therapeutics unrelated to the submitted work. No other authors had disclosures to report. Ethical Approval: This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. The University of Pennsylvania Institutional Review Board deemed this study exempt from review.
Figures

Similar articles
-
Treatment Patterns, Health Care Resource Utilization, and Spending in Medicaid Beneficiaries Initiating Second-generation Long-acting Injectable Agents Versus Oral Atypical Antipsychotics.Clin Ther. 2017 Oct;39(10):1972-1985.e2. doi: 10.1016/j.clinthera.2017.08.008. Epub 2017 Sep 15. Clin Ther. 2017. PMID: 28919292
-
Antipsychotic Adherence and Rehospitalization in Schizophrenia Patients Receiving Oral Versus Long-Acting Injectable Antipsychotics Following Hospital Discharge.J Manag Care Spec Pharm. 2015 Sep;21(9):754-68. doi: 10.18553/jmcp.2015.21.9.754. J Manag Care Spec Pharm. 2015. PMID: 26308223 Free PMC article.
-
Treatment discontinuation of long-acting injectables or oral atypical antipsychotics among Medicaid recipients with schizophrenia.J Med Econ. 2019 Nov;22(11):1105-1112. doi: 10.1080/13696998.2019.1615927. Epub 2019 May 23. J Med Econ. 2019. PMID: 31062998
-
Long-Acting Injections in Schizophrenia: a 3-Year Update on Randomized Controlled Trials Published January 2016-March 2019.Curr Psychiatry Rep. 2019 Nov 19;21(12):124. doi: 10.1007/s11920-019-1114-0. Curr Psychiatry Rep. 2019. PMID: 31745659 Review.
-
Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials.Schizophr Bull. 2014 Jan;40(1):192-213. doi: 10.1093/schbul/sbs150. Epub 2012 Dec 17. Schizophr Bull. 2014. PMID: 23256986 Free PMC article.
References
-
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24. - PubMed
-
- Keepers GA, Fochtmann LJ, Anzia JM, et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2020;177(9):868–72. - PubMed
-
- McDonagh MS, Dana T, Selph S, et al. Treatments for schizophrenia in adults: A systematic review. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

