Restorative Neurostimulation Therapy Compared to Optimal Medical Management: A Randomized Evaluation (RESTORE) for the Treatment of Chronic Mechanical Low Back Pain due to Multifidus Dysfunction
- PMID: 39812968
- PMCID: PMC11751280
- DOI: 10.1007/s40122-024-00689-0
Restorative Neurostimulation Therapy Compared to Optimal Medical Management: A Randomized Evaluation (RESTORE) for the Treatment of Chronic Mechanical Low Back Pain due to Multifidus Dysfunction
Abstract
Introduction: Many interventional strategies are commonly used to treat chronic low back pain (CLBP), though few are specifically intended to target the distinct underlying pathomechanisms causing low back pain. Restorative neurostimulation has been suggested as a specific treatment for mechanical CLBP resulting from multifidus dysfunction. In this randomized controlled trial, we report outcomes from a cohort of patients with CLBP associated with multifidus dysfunction treated with restorative neurostimulation compared to those randomized to a control group receiving optimal medical management (OMM) over 1 year.
Methods: RESTORE is a multicenter, open-label randomized controlled trial. Candidates were assessed for CLBP associated with multifidus dysfunction, with no indication for or history of lumbar spine surgery. Participants were randomized to either restorative neurostimulation with the ReActiv8 system or OMM. The primary endpoint was a comparison of the mean change in the Oswestry Disability Index (ODI) between the treatment and control arms at 1 year, and secondary endpoints included pain (numeric rating scale [NRS]) and health-related quality of life (EuroQol Five-Dimension [EQ-5D-5L]).
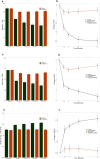
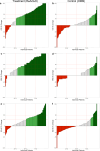
Results: A total of 203 patients, average age 47 years, and with an average 11-year history of low back pain, were included in the analysis. The primary endpoint was a statistically significant demonstration of a clinically relevant mean improvement in the Oswestry Disability Index (ODI) between restorative neurostimulation and OMM arms: ODI (-19.7 ± 1.4 vs. -2.9 ± 1.4; p < 0.001). Additionally, improvements in both the numeric rating scale (NRS) (-3.6 ± 0.2 vs. -0.6 ± 0.2; p < 0.001) and EuroQol Five-Dimension (EQ-5D-5L) (0.155 ± 0.012 vs. 0.008 ± 0.012; p < 0.001) were statistically and clinically significant in the restorative neurostimulation arm compared to the OMM arm.
Conclusion: The RESTORE trial demonstrates that restorative neurostimulation is a safe, reversible, clinically effective, and highly durable option for patients suffering with nonoperative CLBP associated with multifidus dysfunction. This demonstration of treatment superiority over OMM through 1 year is a significant milestone in addressing a major health burden and unmet clinical need.
Trial registration: ClinicalTrials.gov Identifier: NCT04803214.
Keywords: Chronic low back pain; Multifidus dysfunction; Neuromuscular control; Nociceptive pain; Peripheral nerve stimulation; Restorative neurostimulation.
Plain language summary
Chronic low back pain can occur as a consequence of dysfunction in the key stabilizing muscles of the spine, the multifidi. This type of low back pain is difficult to treat, with many interventions resulting in limited improvement or short-term relief for a significant proportion of patients. Despite this limitation, these approaches still represent the best available care in most practices. Restorative neurostimulation is a technique that stimulates dysfunctional multifidi, overriding muscle inhibition to improve spinal function, reduce disability, and alleviate pain. The hypothesis was that this treatment is appropriate for a specific subset of patients who have failed to respond to best available conservative and interventional care. The goal of this study was to compare the effect of restorative neurostimulation to standard-of-care interventions (optimal medical management) for patients with chronic mechanical low back pain. Patients with an average 11-year history of chronic low back pain and diagnosed with multifidus dysfunction were randomly assigned to either ongoing optimal medical management or restorative neurostimulation. At 1 year, disability, pain, and healthcare-related quality of life were assessed. Patients treated with restorative neurostimulation demonstrated significant improvements in their clinical outcomes compared to those receiving optimal medical management alone. Device-related adverse events were rare, reinforcing the safety profile of this technique. This study demonstrated that without restorative neurostimulation, patients with chronic low back pain and multifidus dysfunction have very few effective options and obtained little clinical benefit from ongoing optimal medical management. Restorative neurostimulation is an important advancement for this difficult-to-treat population.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Mainstay Medical (“Mainstay”) funded this trial and compensated all investigators and committee members either directly (consultant fees) or indirectly (payments to institution). Travel expenses related to investigator meetings and training were reimbursed only with prior authorization. Dr. Schwab was the principal investigator for the RESTORE Trial and reports consultant fees and royalties/licenses from Zimmer Biomet, Stryker, and Medtronic, consultant fees from Mainstay Medical and Medicrea, is an executive board member with International Spine Study Group, is a shareholder and has other interests with SeaSpine and VFT Solutions. Dr. K. Patel reports consultant fees from Abbott, Boston Scientific, Biotronik, Mainstay Medical, Vertos, Saluda, SPR Therapeutics, and fiduciary roles as Vice President for the North American Neuromodulation Society and Women Innovators in Pain Medicine, and Director-at-Large for the International Neuromodulation Society. Dr. Langhorst reports consultant fees, honoraria, and meeting support from Mainstay, consultant fees from Vivex, is an advisory/data safety monitoring board member for Vivex, and is a shareholder/holds stock/stock options in Boston Scientific and ATEC Spine. Dr. Heros reports grants, meeting support, and consultant fees from Saluda Medical, grants from Abbott and Ethos Laboratories, meeting support and consultant fees from Mainstay Medical, and consultant fees from Boston Scientific. Dr. Costandi reports grants from Vertos, Medtronic, Vivex, and Saluda. Dr. Moore reports honoraria from Mainstay Medical. Dr. Gilmore reports consultant fees from Mainstay, SPR Therapeutics, Nevro, Nalu, Biotronik, and Saluda, and other interests with SPR Therapeutics. Dr. Chakravarthy reports consultant fees and shareholder/stock options/stock from Mainstay. Dr. Tate reports consultant fees from Nevro, Saluda, Curonix, Vivex, Abbott, Medtronic, and Vertos, honoraria from the Virginia Pain Society and Georgia Society of Interventional Pain Practitioners, board memberships in Women Innovators in Pain Medicine and American Society for Pain and Neuroscience, and secretary of Georgia Society of Interventional Pain Practitioners. Dr. Sanders reports consultant fees and educational event support from Mainstay Medical and Vertos. Dr. Goree reports consultant fees from Saluda, Abbott, and Stratus Medical. Dr. V. Patel reports consultant fees from Mainstay, grants from Orthofix, Pfizer, Premia Spine, Medicrea, Globus, 3-Spine, and Spinal Kinetics, contracts and grants from Aesculap and Medtronic, contracts from Zimmer Biomet Spine, Inc., Johnson & Johnson Medical Device Business Services, NCS America, Simplify Medical, SI Bone, Orthobond Corp., Cerapedics, consultant fees from Spine Welding, SI Bone, expert testimony for Ogborn Mihm, LLP Expert Witness Deposition, and educational event support from Ecential Robotics and Johnson & Johnson Medical. Dr. Lehmen reports consultant fees, honoraria, and meeting support from Mainstay and Globus Medical-NuVasive. Dr. Desai reports royalties/licenses from Nevro, consultant fees from Medtronic, Nalu, and SPR Therapeutics, data safety monitoring/advisory board membership with FUSMobile, shareholder/stock/stock options in HypreVention, SPR Therapeutics, SynerFuse, and Virdio Health. Dr. Pope reports consulting fees, honoraria, research grants, educational support, and shareholder/stock options/stock from Abbott and Saluda, research grants, honoraria, royalties/license, consulting fees and shareholder/stock options/stock from Aurora, research grant from AIS, research grants, consulting fees, and honoraria from Boston Scientific, Flowonix, Ethos, Mainstay Medical, Muse, grants, consulting fees, honoraria, and shareholder/stock options/stock from Painteq, SPR Therapeutics, Theraquil, Vertos, consulting fees and honoraria from Medtronic, Tersera, Vertiflex, and WISE, consulting fees, honoraria, and shareholder/stock options/stock from Spark and SpineThera, shareholder/stock options/stock from Anesthetic Gas Reclamation, Axonics, Celeri Health, Pacific Research Institute, and Stimgenics, royalties/licenses and shareholder/stock options/stock from Neural Integrative Solutions, royalties/licenses from Elsevier and Springer, a patent for Neuronmonitoring, and fiduciary roles as Chairman for Pacific Spine and Pain Society and immediate past president of the American Society for Pain and Neuroscience. Dr. Giuffrida reports consultant fees, medical writing support and a medical advisory board membership from Mainstay, and executive board membership for the American Society of Pain and Neuroscience. Dr. Hayek reports payment for expert witness defense testimony, data safety/monitoring board membership on three non-industry funded studies, and was 2022–2023 past president of North American Neuromodulation Society. Dr. Virk reports research/educational event support from Mainstay, and consultant fees and research support from LifeSpine. Dr. Paicius reports consulting fees from Abbott and Biotronik; Dr. Mekhail functioned as independent medical monitor of the RESTORE trial has a consultancy agreement with Mainstay Medical. Dr. Levy reports unpaid consultancies with Abbott, Biotronik, Nalu, and Saluda, and stock options with Nalu and Saluda. Dr. Gilligan reports consultant fees from Mainstay Medical, Saluda, Persica, Iliad Lifesciences, and Biotronik, expert witness testimony payment, and fiduciary roles as Editor in Chief of Pain Practice, finance committee member for North American Neuromodulation Society, and on the board of directors for International Neuromodulation Society. Drs. Gentile, Bundy, Manion, and Klemme report consultant fees from Mainstay Medical. Drs. Meyer, Vaid, and Szentirmai have no other disclosures. Ethical Approval: The RESTORE trial followed the principles of the Declaration of Helsinki of 1964 and its later amendments and Good Clinical Practice (GCP). The WCG IRB acted as the Central IRB (RN#20,211,219) for most sites while other sites had local IRB approval prior to enrollment of patients. All patients provided written informed consent to participate in the trial.
Figures
References
-
- Kreiner DS, Matz P, Bono CM, Cho CH, Easa JE, Ghiselli G, et al. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of low back pain. Spine J. 2020;20:998–1024. - PubMed
-
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403:2133–61. - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

