Blood Culture Use in Medical and Surgical Intensive Care Units and Wards
- PMID: 39813030
- PMCID: PMC11736503
- DOI: 10.1001/jamanetworkopen.2024.54738
Blood Culture Use in Medical and Surgical Intensive Care Units and Wards
Abstract
Importance: Blood culture (BC) use benchmarks in US hospitals have not been defined.
Objective: To characterize BC use in adult intensive care units (ICUs) and wards in US hospitals.
Design, setting, and participants: A retrospective cross-sectional study of BC use in adult medical ICUs, medical-surgical ICUs, medical wards, and medical-surgical wards from acute care hospitals from the 4 US geographic regions was conducted. Critical access hospitals, less than 6 months of BC data, and non-US hospitals were excluded. The study included BC use data from September 1, 2019, to August 31, 2021. Data were analyzed from February 23 to July 14, 2024.
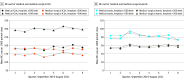
Main outcomes and measures: The primary outcome was BC use per 1000 patient-days. Adjusted means with 95% CIs were calculated using mixed-effects negative binomial regression models adjusted for unit type, hospital bed size, geographic region, seasonality, and state COVID-19 case load, with random intercepts accounting for clustering at unit and hospital levels. Secondary outcomes included blood culture positivity, single BCs, BC contamination, and minimum threshold for BC use where blood culture positivity would be optimized.
Results: A total of 362 327 blood cultures were analyzed from 27 medical ICUs, 35 medical-surgical ICUs, 121 medical wards, and 109 medical-surgical wards from 48 hospitals in 19 states and the District of Columbia. The adjusted mean BC use per 1000 patient-days was 273.1 (95% CI, 270.2-275.9) for medical ICUs, 146.0 (95% CI, 144.5-147.5) for medical-surgical ICUs, 80.3 (95% CI, 79.8-80.7) for medical wards, and 65.1 for medical-surgical wards. Blood culture use was significantly higher across all 4 unit types in hospitals with more than 500 beds compared with 500 or less beds and in the West-Midwest compared with other regions. Single blood culture and positive blood culture rates were below 10% across all 4 unit types. Of the 292 units, 97% had a mean BC contamination rate within 3% of the recommended threshold, and 51% were within 1%. The minimum BC use thresholds (ie, BC use below this number may represent undertesting) were 120 BCs per 1000 patient-days for medical ICUs, 80 BCs per 1000 patient-days for medical-surgical ICUs, and 30 BCs per 1000 patient-days for medical-surgical wards.
Conclusions and relevance: The findings of this study suggest that blood culture positivity may help determine appropriate BC use for individual unit types.
Conflict of interest statement
Figures
References
-
- Dräger S, Giehl C, Søgaard KK, et al. Do we need blood culture stewardship programs? a quality control study and survey to assess the appropriateness of blood culture collection and the knowledge and attitudes among physicians in Swiss hospitals. Eur J Intern Med. 2022;103:50-56. doi: 10.1016/j.ejim.2022.04.028 - DOI - PubMed
-
- Doern GV, Carroll KC, Diekema DJ, et al. Practical guidance for clinical microbiology laboratories: a comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clin Microbiol Rev. 2019;33(1):e00009-19. doi: 10.1128/CMR.00009-19 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

