The Golgi complex governs natural killer cell lytic granule positioning to promote directionality in cytotoxicity
- PMID: 39813120
- PMCID: PMC11844255
- DOI: 10.1016/j.celrep.2024.115156
The Golgi complex governs natural killer cell lytic granule positioning to promote directionality in cytotoxicity
Abstract
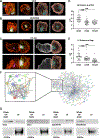
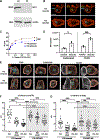
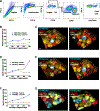
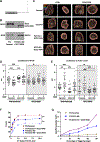
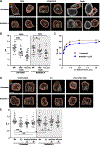
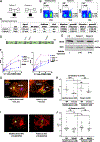
Cytotoxic immune cells mediate precise attacks against diseased cells to maintain organismal health. Their operational unit of killing and host defense is lytic granules (LGs), which are specialized lysosomal-related organelles. Precision in cytotoxicity is achieved by converging the many LGs to the microtubule-organizing center (MTOC) and polarizing these to the diseased cell for secretion. We identify unappreciated intimate relationships between the Golgi, MTOC, and LGs after cytotoxic cell activation, as well as the trans-Golgin protein GCC2 on the LG surface. GCC2 serves to tether LGs to the Golgi following convergence, and both GCC2 and the Golgi are required for the persistence of convergence. GCC2 allows LGs to utilize the Golgi as a docking station preventing LG dispersion and innocent bystander killing in complex three-dimensional environments. We also identify GCC2 variants causing human natural killer cell deficiency, further emphasizing the importance of LG convergence and Golgi linkage in precision targeting for human immunity.
Keywords: CP: Immunology; GCC2; Golgi; Golgins; cancer; confocal microscopy; cytotoxicity; lytic granule convergence; natural killer cells.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.A.P., F.v.d.H., Y.L., and J.S.O. are inventors on a patent application (applied through the Trustees of Columbia University in the city of New York, application number PCT/US2023/01614, entitled “Compounds, targets, and methods for modulating lytic granule convergence in cytotoxic cells to promote bystander killing in cellular therapies”). J.R.L. has stock ownership in 23andMe and is a co-inventor on multiple patents related to molecular diagnostics for inherited neuropathies, eye diseases, genomic disorders, and bacterial genomic fingerprinting.
Figures

Similar articles
-
Rapid activation receptor- or IL-2-induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling.Blood. 2013 Apr 4;121(14):2627-37. doi: 10.1182/blood-2012-06-437012. Epub 2013 Feb 4. Blood. 2013. PMID: 23380740 Free PMC article.
-
Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment.Mol Biol Cell. 2010 Jul 1;21(13):2241-56. doi: 10.1091/mbc.e09-11-0930. Epub 2010 May 5. Mol Biol Cell. 2010. PMID: 20444980 Free PMC article.
-
Locked and Loaded: Mechanisms Regulating Natural Killer Cell Lytic Granule Biogenesis and Release.Front Immunol. 2022 Apr 26;13:871106. doi: 10.3389/fimmu.2022.871106. eCollection 2022. Front Immunol. 2022. PMID: 35558071 Free PMC article. Review.
-
Measurement of Lytic Granule Convergence After Formation of an NK Cell Immunological Synapse.Methods Mol Biol. 2017;1584:497-515. doi: 10.1007/978-1-4939-6881-7_31. Methods Mol Biol. 2017. PMID: 28255722 Free PMC article.
-
Molecular regulation of the plasma membrane-proximal cellular steps involved in NK cell cytolytic function.J Cell Sci. 2020 Feb 21;133(5):jcs240424. doi: 10.1242/jcs.240424. J Cell Sci. 2020. PMID: 32086255 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

