Intranasal Delivery of a Ghrelin Mimetic Engages the Brain Ghrelin Signaling System in Mice
- PMID: 39813130
- PMCID: PMC11795113
- DOI: 10.1210/endocr/bqae166
Intranasal Delivery of a Ghrelin Mimetic Engages the Brain Ghrelin Signaling System in Mice
Abstract
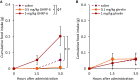
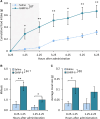
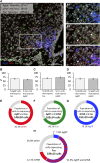
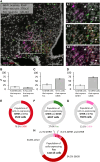
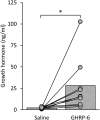
Ghrelin, the endogenous ligand of the growth hormone secretagogue receptor (GHSR), promotes food intake and other feeding behaviors, and stimulates growth hormone (GH) release from the pituitary. Growth hormone secretagogues (GHS), such as GHRP-6 and MK-0677, are synthetic GHSR ligands that activate orexigenic neuropeptide Y neurons that coexpress agouti-related peptide (AgRP) in the arcuate nucleus of the hypothalamus when administered systemically. Systemic GHRP-6 also stimulates GH release in humans and rats. Thus, GHS and ghrelin have therapeutic relevance in patients who could benefit from its orexigenic and/or GH-releasing effects. This study examined whether intranasal delivery of ghrelin, GHRP-6, or MK-0677 engages the brain ghrelin signaling system. Effective compounds and doses were selected based on increased food intake after intranasal application in mice. Only GHRP-6 (5 mg/kg) increased food intake without adverse effects, prompting detailed analysis of meal patterns, neuronal activation in the arcuate nucleus (via Fos mapping) and neurochemical identification of c-fos messenger RNA (mRNA)-expressing neurons using RNAscope. We also assessed the effect of intranasal GHRP-6 on serum GH levels. Intranasal GHRP-6 increased food intake by increasing meal frequency and size. Fos expression in the arcuate nucleus was higher in GHRP-6-treated mice than in saline controls. When examining the neurochemical identity of c-fos-mRNA-expressing neurons, we found coexpression with 63.5 ± 1.9% Ghsr mRNA, 79 ± 6.8% Agrp mRNA, and 11.4 ± 2.5% Ghrh mRNA, demonstrating GHRP-6's ability to engage arcuate nucleus neurons involved in food intake and GH release. Additionally, intranasal GHRP-6 elevated GH serum levels. These findings suggest that intranasal GHRP-6, but not ghrelin or MK-0677, can engage the brain ghrelin signaling system.
Keywords: GHSR; arcuate nucleus; food intake; ghrelin receptor agonist; growth hormone; intranasal.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656‐660. - PubMed
-
- de la Cour CD, Norlén P, Håkanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regul Pept. 2007;143(1-3):118‐126. - PubMed
-
- Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974‐977. - PubMed
-
- Bowers CY, Momany FA, Reynolds GA, Hong A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology. 1984;114(5):1537‐1545. - PubMed
-
- Smith RG, Cheng K, Schoen WR, et al. A nonpeptidyl growth hormone secretagogue. Science. 1993;260(5114):1640‐1643. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

