Long-term survival of participants in the PASART-1 and PASART-2 trials of neo-adjuvant pazopanib and radiotherapy in soft tissue sarcoma
- PMID: 39813174
- PMCID: PMC11758146
- DOI: 10.2340/1651-226X.2025.42333
Long-term survival of participants in the PASART-1 and PASART-2 trials of neo-adjuvant pazopanib and radiotherapy in soft tissue sarcoma
Abstract
Objective: This study aims to assess the long-term safety and efficacy of adding pazopanib to neo-adjuvant radiotherapy followed by surgery in patients with high-risk non-metastatic soft tissue sarcoma of the trunk and extremities treated in the PASART-1 and PASART-2 trials, as well as to compare the PASART cohorts to a control cohort receiving standard treatment during the same time period from the Netherlands Cancer Registry (IKNL) to investigate if adding pazopanib improves Overall Survival (OS).
Methods: Updated follow-up data on disease control, survival and long-term toxicities of the PASART-trials were extracted from electronic patient records. The effect of adding pazopanib to neo-adjuvant radiotherapy on OS was investigated by comparing the combined PASART cohorts to the IKNL cohort via direct comparison and exact matching analysis.
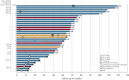
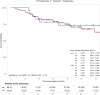
Results: PASART-trials included 34 patients, IKNL cohort included 487 patients. After a median follow-up of 75.4 months (range: 30-131 months) the 1-year, 2-year and 5-year OS in the PASART-trials were 97% (95% confidence interval [CI]: 91.5-100), 85.3% (95% CI: 74.2-98.1), 79.3% (95% CI: 66.8-94.2), respectively. Matching resulted in 23 PASART and 89 IKNL patients. Adding pazopanib did not significantly improve OS when compared to standard treatment (IKNL) in a direct comparison (hazard ratio [HR]: 0.58; 95% CI: 0.30-1.13) or matched analysis (HR: 0.70; 95% CI: 0.29-1.73). Long-term toxicities, mainly fibrosis (n = 6) and edema (n = 2), were observed in 11 PASART patients and comparable to historical controls.
Interpretation: The addition of pazopanib had tolerable long-term toxicity but did not improve OS when compared to a control cohort receiving standard treatment.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures
Similar articles
-
A phase II study on the neo-adjuvant combination of pazopanib and radiotherapy in patients with high-risk, localized soft tissue sarcoma.Acta Oncol. 2021 Dec;60(12):1557-1564. doi: 10.1080/0284186X.2021.1971294. Epub 2021 Sep 23. Acta Oncol. 2021. PMID: 34554030 Clinical Trial.
-
Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): a multicentre, randomised, open-label, phase 2 trial.Lancet Oncol. 2020 Aug;21(8):1110-1122. doi: 10.1016/S1470-2045(20)30325-9. Epub 2020 Jul 20. Lancet Oncol. 2020. PMID: 32702309 Free PMC article. Clinical Trial.
-
Long-term responders and survivors on pazopanib for advanced soft tissue sarcomas: subanalysis of two European Organisation for Research and Treatment of Cancer (EORTC) clinical trials 62043 and 62072.Ann Oncol. 2014 Mar;25(3):719-724. doi: 10.1093/annonc/mdt586. Epub 2014 Feb 6. Ann Oncol. 2014. PMID: 24504442 Free PMC article. Clinical Trial.
-
Vascular endothelial growth factor (VEGF) targeting therapy for persistent, recurrent, or metastatic cervical cancer.Cochrane Database Syst Rev. 2021 Mar 4;3(3):CD013348. doi: 10.1002/14651858.CD013348.pub2. Cochrane Database Syst Rev. 2021. PMID: 33661538 Free PMC article.
-
Pazopanib, a new therapy for metastatic soft tissue sarcoma.Expert Opin Pharmacother. 2013 May;14(7):929-35. doi: 10.1517/14656566.2013.780030. Epub 2013 Mar 14. Expert Opin Pharmacother. 2013. PMID: 23488774 Review.
References
-
- Haas RL, Miah AB, LePechoux C, DeLaney TF, Baldini EH, Alektiar K, et al. . Preoperative radiotherapy for extremity soft tissue sarcoma; past, present and future perspectives on dose fractionation regimens and combined modality strategies. Radiother Oncol. 2016;119(1):14–21. 10.1016/j.radonc.2015.12.002 - DOI - PMC - PubMed
-
- Blay JY, Le Cesne A, Penel N, Bompas E, Chevreau C, Duffaud F, et al. . 1397O – the nationwide cohort of 26,883 patients with sarcomas treated in NETSARC reference network between 2010 and 2015 in France: major impact of multidisciplinary board presentation prior to 1st treatment. Ann Oncol. 2016;27:vi483. 10.1093/annonc/mdw388.03 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

