Soil microbiome bacteria protect plants against filamentous fungal infections via intercellular contacts
- PMID: 39813250
- PMCID: PMC11762177
- DOI: 10.1073/pnas.2418766122
Soil microbiome bacteria protect plants against filamentous fungal infections via intercellular contacts
Abstract
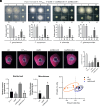
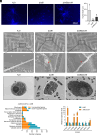
Bacterial-fungal interaction (BFI) has significant implications for the health of host plants. While the diffusible antibiotic metabolite-mediated competition in BFI has been extensively characterized, the impact of intercellular contact remains largely elusive. Here, we demonstrate that the intercellular contact is a prevalent mode of interaction between beneficial soil bacteria and pathogenic filamentous fungi. By generating antibiotics-deficient mutants in two common soil bacteria, Lysobacter enzymogenes and Pseudomonas fluorescens, we show that antibiotics-independent BFI effectively inhibits pathogenic fungi. Furthermore, transcriptional and genetic evidence revealed that this antibiotics-independent BFI relies on intercellular contact mediated by the type VI secretion system (T6SS), which may facilitate the translocation of bacterial toxic effectors into fungal cells. Finally, by using a "conidia enrichment" platform, we found that T6SS-mediated fungal inhibition resulting from intercellular contact naturally occurs within the soil microbiome, particularly represented by Pseudomonas fulva. Overall, these results demonstrate that bacteria from the soil microbiome can protect host plants from fungal infection through antibiotics-independent intercellular contacts, thus revealing a naturally occurring and ecologically important mode of BFI in agricultural contexts.
Keywords: T6SS; bacterial–fungal interaction; contact-dependent antifungal activity; filamentous fungi.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Avery S. V., Singleton I., Magan N., Goldman G. H., The fungal threat to global food security. Fungal Biol. 123, 555–557 (2019). - PubMed
-
- Fisher M. C., Hawkins N. J., Sanglard D., Gurr S. J., Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360, 739–742 (2018). - PubMed
-
- Zhan C., Matsumoto H., Liu Y., Wang M., Pathways to engineering the phyllosphere microbiome for sustainable crop production. Nat. Food 3, 997–1004 (2022). - PubMed
-
- Fira D., Dimkic I., Beric T., Lozo J., Stankovic S., Biological control of plant pathogens by Bacillus species. J. Biotechnol. 285, 44–55 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

