Gene therapy ameliorates bowel dysmotility and enteric neuron degeneration and extends survival in lysosomal storage disorder mouse models
- PMID: 39813314
- PMCID: PMC12087356
- DOI: 10.1126/scitranslmed.adj1445
Gene therapy ameliorates bowel dysmotility and enteric neuron degeneration and extends survival in lysosomal storage disorder mouse models
Abstract
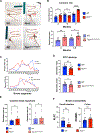
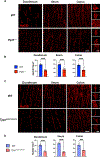
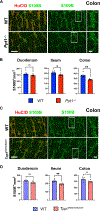
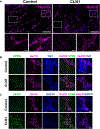
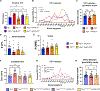
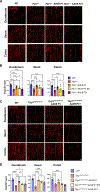
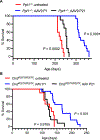
Children with neurodegenerative disease often have debilitating gastrointestinal symptoms. We hypothesized that this may be due at least in part to underappreciated degeneration of neurons in the enteric nervous system (ENS), the master regulator of bowel function. To test this hypothesis, we evaluated mouse models of neuronal ceroid lipofuscinosis type 1 and 2 (CLN1 and CLN2 disease, respectively), neurodegenerative lysosomal storage disorders caused by deficiencies in palmitoyl protein thioesterase-1 and tripeptidyl peptidase-1, respectively. Both mouse lines displayed slow bowel transit in vivo that worsened with age. Although the ENS appeared to develop normally in these mice, there was a progressive and profound loss of myenteric plexus neurons accompanied by changes in enteric glia in adult mice. Similar pathology was evident in colon autopsy material from a child with CLN1 disease. Neonatal administration of adeno-associated virus-mediated gene therapy prevented bowel transit defects, ameliorated loss of enteric neurons, and extended survival in mice. Treatment after weaning was less effective than treating neonatally but still extended the lifespan of CLN1 disease mice. These data provide proof-of-principle evidence of ENS degeneration in two lysosomal storage diseases and suggest that gene therapy can ameliorate ENS disease, also improving survival.
Conflict of interest statement
Figures

References
-
- Bhardwaj J, Wan DQ, Koenig MK, Liu Y, Hashmi SS, Rhoads JM, Impaired gastric emptying and small bowel transit in children with mitochondrial disorders. J. Pediatr. Gastroenterol. Nutr. 55, 194–199 (2012). - PubMed
-
- Dinari G, Rosenbach Y, Grunebaum M, Zahavi I, Alpert G, Nitzan M, Gastrointestinal manifestations of Niemann-Pick disease. Enzyme 25, 407–412 (1980). - PubMed
-
- Fagerberg CR, Taylor A, Distelmaier F, Schrøder HD, Kibæk M, Wieczorek D, Tarnopolsky M, Brady L, Larsen MJ, Jamra RA, Seibt A, Hejbøl EK, Gade E, Markovic L, Klee D, Nagy P, Rouse N, Agarwal P, Dolinsky VW, Bakovic M, Choline transporter-like 1 deficiency causes a new type of childhood-onset neurodegeneration. Brain 143, 94–111 (2020). - PubMed
-
- Finsterer J, Leigh and leigh-like syndrome in children and adults. Pediatr. Neurol. 39, 223–235 (2008). - PubMed
-
- Thapar N, Saliakellis E, Benninga MA, Borrelli O, Curry J, Faure C, De Giorgio R, Gupte G, Knowles CH, Staiano A, Vandenplas Y, Di Lorenzo C, Paediatric intestinal pseudo-obstruction: Evidence and consensus-based recommendations from an ESPGHAN-led expert group. J. Pediatr. Gastroenterol. Nutr. 66, 991–1019 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

